High expression of KCa3.1 in patients with clear cell renal carcinoma predicts high metastatic risk and poor survival
- PMID: 25848765
- PMCID: PMC4388734
- DOI: 10.1371/journal.pone.0122992
High expression of KCa3.1 in patients with clear cell renal carcinoma predicts high metastatic risk and poor survival
Abstract
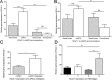
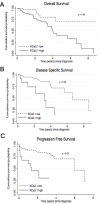
Background: Ca2+-activated K+ channels have been implicated in cancer cell growth, metastasis, and tumor angiogenesis. Here we hypothesized that high mRNA and protein expression of the intermediate-conductance Ca2+-activated K+ channel, KCa3.1, is a molecular marker of clear cell Renal Cell Carcinoma (ccRCC) and metastatic potential and survival.
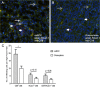
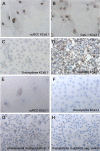
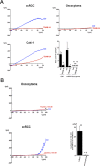
Methodology/principal findings: We analyzed channel expression by qRT-PCR, immunohistochemistry, and patch-clamp in ccRCC and benign oncocytoma specimens, in primary ccRCC and oncocytoma cell lines, as well as in two ccRCC cell lines (Caki-1 and Caki-2). CcRCC specimens contained 12-fold higher mRNA levels of KCa3.1 than oncocytoma specimens. The large-conductance channel, KCa1.1, was 3-fold more highly expressed in ccRCC than in oncocytoma. KCa3.1 mRNA expression in ccRCC was 2-fold higher than in the healthy cortex of the same kidney. Disease specific survival trended towards reduction in the subgroup of high-KCa3.1-expressing tumors (p<0.08 vs. low-KCa3.1-expressing tumors). Progression-free survival (time to metastasis/recurrence) was reduced significantly in the subgroup of high-KCa3.1-expressing tumors (p<0.02, vs. low-KCa3.1-expressing tumors). Immunohistochemistry revealed high protein expression of KCa3.1 in tumor vessels of ccRCC and oncocytoma and in a subset of ccRCC cells. Oncocytoma cells were devoid of KCa3.1 protein. In a primary ccRCC cell line and Caki-1/2-ccRCC cells, we found KCa3.1-protein as well as TRAM-34-sensitive KCa3.1-currents in a subset of cells. Furthermore, Caki-1/2-ccRCC cells displayed functional Paxilline-sensitive KCa1.1 currents. Neither KCa3.1 nor KCa1.1 were found in a primary oncocytoma cell line. Yet KCa-blockers, like TRAM-34 (KCa3.1) and Paxilline (KCa1.1), had no appreciable effects on Caki-1 proliferation in-vitro.
Conclusions/significance: Our study demonstrated expression of KCa3.1 in ccRCC but not in benign oncocytoma. Moreover, high KCa3.1-mRNA expression levels were indicative of low disease specific survival of ccRCC patients, short progression-free survival, and a high metastatic potential. Therefore, KCa3.1 is of prognostic value in ccRCC.
Conflict of interest statement
Figures


References
-
- Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H et al. (2005) International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57: 463–472. - PubMed
-
- Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V (2007) Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem 14: 1437–1457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

