Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals
- PMID: 25848808
- PMCID: PMC5812269
- DOI: 10.1021/acs.nanolett.5b01254
Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals
Abstract
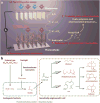
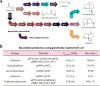
Direct solar-powered production of value-added chemicals from CO2 and H2O, a process that mimics natural photosynthesis, is of fundamental and practical interest. In natural photosynthesis, CO2 is first reduced to common biochemical building blocks using solar energy, which are subsequently used for the synthesis of the complex mixture of molecular products that form biomass. Here we report an artificial photosynthetic scheme that functions via a similar two-step process by developing a biocompatible light-capturing nanowire array that enables a direct interface with microbial systems. As a proof of principle, we demonstrate that a hybrid semiconductor nanowire-bacteria system can reduce CO2 at neutral pH to a wide array of chemical targets, such as fuels, polymers, and complex pharmaceutical precursors, using only solar energy input. The high-surface-area silicon nanowire array harvests light energy to provide reducing equivalents to the anaerobic bacterium, Sporomusa ovata, for the photoelectrochemical production of acetic acid under aerobic conditions (21% O2) with low overpotential (η < 200 mV), high Faradaic efficiency (up to 90%), and long-term stability (up to 200 h). The resulting acetate (∼6 g/L) can be activated to acetyl coenzyme A (acetyl-CoA) by genetically engineered Escherichia coli and used as a building block for a variety of value-added chemicals, such as n-butanol, polyhydroxybutyrate (PHB) polymer, and three different isoprenoid natural products. As such, interfacing biocompatible solid-state nanodevices with living systems provides a starting point for developing a programmable system of chemical synthesis entirely powered by sunlight.
Keywords: Nanowires; artificial photosynthesis; bacteria; carbon dioxide fixation.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

