Transition States and transition state analogue interactions with enzymes
- PMID: 25848811
- PMCID: PMC4482137
- DOI: 10.1021/acs.accounts.5b00002
Transition States and transition state analogue interactions with enzymes
Abstract
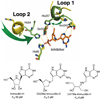
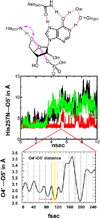
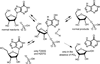
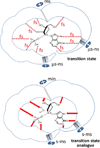
Enzymatic transition states have lifetimes of a few femtoseconds (fs). Computational analysis of enzyme motions leading to transition state formation suggests that local catalytic site motions on the fs time scale provide the mechanism to locate transition states. An experimental test of protein fs motion and its relation to transition state formation can be provided by isotopically heavy proteins. Heavy enzymes have predictable mass-altered bond vibration states without altered electrostatic properties, according to the Born-Oppenheimer approximation. On-enzyme chemistry is slowed in most heavy proteins, consistent with altered protein bond frequencies slowing the search for the transition state. In other heavy enzymes, structural changes involved in reactant binding and release are also influenced. Slow protein motions associated with substrate binding and catalytic site preorganization are essential to allow the subsequent fs motions to locate the transition state and to facilitate the efficient release of products. In the catalytically competent geometry, local groups move in stochastic atomic motion on the fs time scale, within transition state-accessible conformations created by slower protein motions. The fs time scale for the transition state motions does not permit thermodynamic equilibrium between the transition state and stable enzyme states. Isotopically heavy enzymes provide a diagnostic tool for fast coupled protein motions to transition state formation and mass-dependent conformational changes. The binding of transition state analogue inhibitors is the opposite in catalytic time scale to formation of the transition state but is related by similar geometries of the enzyme-transition state and enzyme-inhibitor interactions. While enzymatic transition states have lifetimes as short as 10(-15) s, transition state analogues can bind tightly to enzymes with release rates greater than 10(3) s. Tight-binding transition state analogues stabilize the rare but evolved enzymatic geometry to form the transition state. Evolution to efficient catalysis optimized this geometry and its stabilization by a transition state mimic results in tight binding. Release rates of transition state analogues are orders of magnitude slower than product release in normal catalytic function. During catalysis, product release is facilitated by altered chemistry. Compared to the weak associations found in Michaelis complexes, transition state analogues involve strong interactions related to those in the transition state. Optimum binding of transition state analogues occurs when the complex retains the system motions intrinsic to transition state formation. Conserved dynamic motion retains the entropic components of inhibitor complexes, improving the thermodynamics of analogue binding.
Figures


References
-
- Knowles JR. Enzyme Catalysis: Not Different, Just Better. Nature. 1991;350:121–124. - PubMed
-
- Basner JE, Schwartz SD. How Enzyme Dynamics Helps Catalyze a Reaction in Atomic Detail: A Transition Path Sampling Study. J. Am. Chem. Soc. 2005;127:13822–13831. - PubMed
-
- Bolhuis PG, Chandler D, Dellago C, Geissler PL. Transition Path Sampling: Throwing Ropes over Rough Mountain Passes, in the Dark. Annu. Rev. Phys. Chem. 2002;53:291–318. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

