Local origin of mesenchymal cells in a murine orthotopic lung transplantation model of bronchiolitis obliterans
- PMID: 25848843
- PMCID: PMC4450312
- DOI: 10.1016/j.ajpath.2015.03.002
Local origin of mesenchymal cells in a murine orthotopic lung transplantation model of bronchiolitis obliterans
Abstract
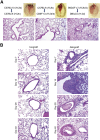
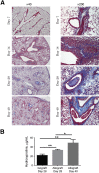
Bronchiolitis obliterans is the leading cause of chronic graft failure and long-term mortality in lung transplant recipients. Here, we used a novel murine model to characterize allograft fibrogenesis within a whole-lung microenvironment. Unilateral left lung transplantation was performed in mice across varying degrees of major histocompatibility complex mismatch combinations. B6D2F1/J (a cross between C57BL/6J and DBA/2J) (Haplotype H2b/d) lungs transplanted into DBA/2J (H2d) recipients were identified to show histopathology for bronchiolitis obliterans in all allogeneic grafts. Time course analysis showed an evolution from immune cell infiltration of the bronchioles and vessels at day 14, consistent with acute rejection and lymphocytic bronchitis, to subepithelial and intraluminal fibrotic lesions of bronchiolitis obliterans by day 28. Allografts at day 28 showed a significantly higher hydroxyproline content than the isografts (33.21 ± 1.89 versus 22.36 ± 2.33 μg/mL). At day 40 the hydroxyproline content had increased further (48.91 ± 7.09 μg/mL). Flow cytometric analysis was used to investigate the origin of mesenchymal cells in fibrotic allografts. Collagen I-positive cells (89.43% ± 6.53%) in day 28 allografts were H2Db positive, showing their donor origin. This novel murine model shows consistent and reproducible allograft fibrogenesis in the context of single-lung transplantation and represents a major step forward in investigating mechanisms of chronic graft failure.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
A relevant experimental model for human bronchiolitis obliterans syndrome.J Heart Lung Transplant. 2013 Nov;32(11):1131-9. doi: 10.1016/j.healun.2013.07.016. Epub 2013 Sep 16. J Heart Lung Transplant. 2013. PMID: 24050896
-
The mitigating effect of exogenous carbon monoxide on chronic allograft rejection and fibrosis post-lung transplantation.J Heart Lung Transplant. 2023 Mar;42(3):317-326. doi: 10.1016/j.healun.2022.11.005. Epub 2022 Nov 26. J Heart Lung Transplant. 2023. PMID: 36522238
-
Role of airway epithelial injury in murine orthotopic tracheal allograft rejection.Ann Thorac Surg. 2006 Oct;82(4):1226-33. doi: 10.1016/j.athoracsur.2006.03.122. Ann Thorac Surg. 2006. PMID: 16996912
-
Small animal models of experimental obliterative bronchiolitis.Am J Respir Cell Mol Biol. 2013 Jun;48(6):675-84. doi: 10.1165/rcmb.2012-0379TR. Am J Respir Cell Mol Biol. 2013. PMID: 23392572 Review.
-
Human and murine obliterative bronchiolitis in transplant.Proc Am Thorac Soc. 2007 Jan;4(1):37-43. doi: 10.1513/pats.200605-107JG. Proc Am Thorac Soc. 2007. PMID: 17202290 Free PMC article. Review.
Cited by
-
Models of Lung Transplant Research: a consensus statement from the National Heart, Lung, and Blood Institute workshop.JCI Insight. 2017 May 4;2(9):e93121. doi: 10.1172/jci.insight.93121. eCollection 2017 May 4. JCI Insight. 2017. PMID: 28469087 Free PMC article. Review.
-
Vitamin E stabilizes iron and mitochondrial metabolism in pulmonary fibrosis.Front Pharmacol. 2023 Dec 6;14:1240829. doi: 10.3389/fphar.2023.1240829. eCollection 2023. Front Pharmacol. 2023. PMID: 38125893 Free PMC article.
-
Anti-CD20 Antibody and Calcineurin Inhibitor Combination Therapy Effectively Suppresses Antibody-Mediated Rejection in Murine Orthotopic Lung Transplantation.Life (Basel). 2023 Oct 11;13(10):2042. doi: 10.3390/life13102042. Life (Basel). 2023. PMID: 37895424 Free PMC article.
-
Cross-Regulation of F-Box Protein FBXL2 with T-bet and TNF-α during Acute and Chronic Lung Allograft Rejection.J Immunol. 2022 Nov 1;209(9):1788-1795. doi: 10.4049/jimmunol.2200245. Epub 2022 Sep 16. J Immunol. 2022. PMID: 36113884 Free PMC article.
-
A decline in club cell secretory proteins in lung transplantation is associated with release of natural killer cells exosomes leading to chronic rejection.J Heart Lung Transplant. 2021 Dec;40(12):1517-1528. doi: 10.1016/j.healun.2021.08.016. Epub 2021 Sep 15. J Heart Lung Transplant. 2021. PMID: 34627707 Free PMC article.
References
-
- Barker A.F., Bergeron A., Rom W.N., Hertz M.I. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. - PubMed
-
- Estenne M., Hertz M.I. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–444. - PubMed
-
- Estenne M., Maurer J.R., Boehler A., Egan J.J., Frost A., Hertz M., Mallory G.B., Snell G.I., Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. - PubMed
-
- Scott A.I., Sharples L.D., Stewart S. Bronchiolitis obliterans syndrome: risk factors and therapeutic strategies. Drugs. 2005;65:761–771. - PubMed
-
- Sharples L.D., McNeil K., Stewart S., Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21:271–281. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

