Protein palmitoylation is critical for the polar growth of root hairs in Arabidopsis
- PMID: 25849075
- PMCID: PMC4340681
- DOI: 10.1186/s12870-015-0441-5
Protein palmitoylation is critical for the polar growth of root hairs in Arabidopsis
Abstract
Background: Protein palmitoylation, which is critical for membrane association and subcellular targeting of many signaling proteins, is catalyzed mainly by protein S-acyl transferases (PATs). Only a few plant proteins have been experimentally verified to be subject to palmitoylation, such as ROP GTPases, calcineurin B like proteins (CBLs), and subunits of heterotrimeric G proteins. However, emerging evidence from palmitoyl proteomics hinted that protein palmitoylation as a post-translational modification might be widespread. Nonetheless, due to the large number of genes encoding PATs and the lack of consensus motifs for palmitoylation, progress on the roles of protein palmitoylation in plants has been slow.
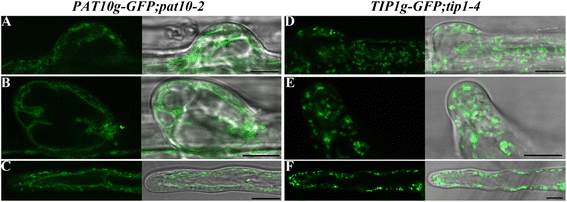
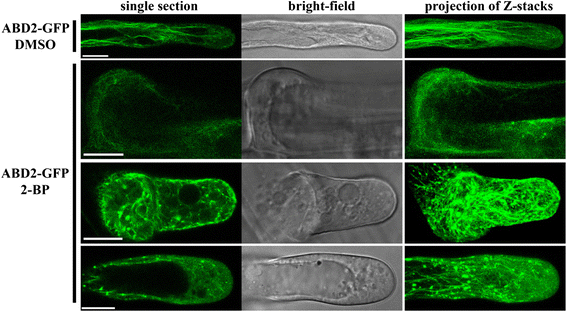
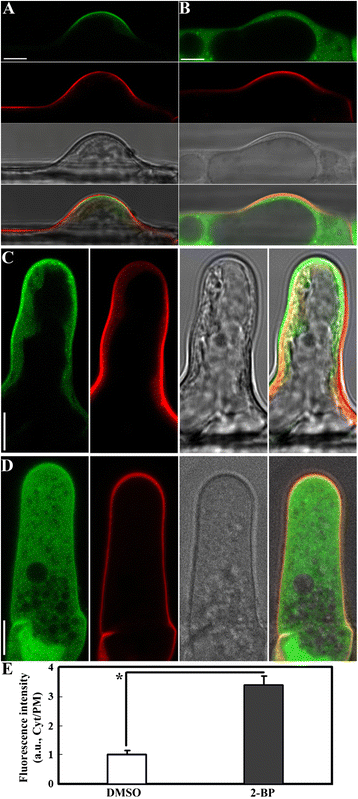
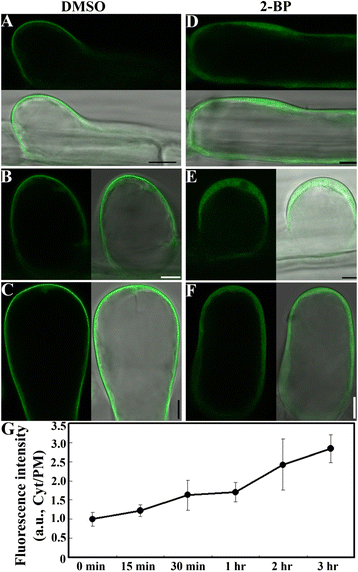
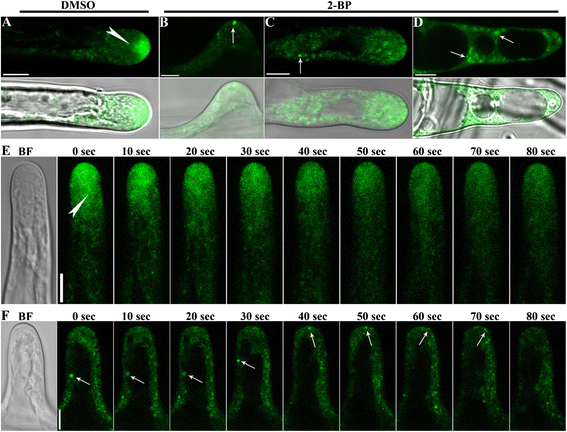
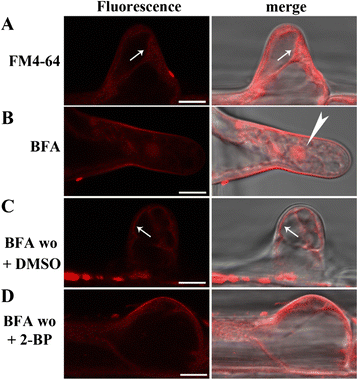
Results: We combined pharmacological and genetic approaches to examine the role of protein palmitoylation in root hair growth. Multiple PATs from different endomembrane compartments may participate in root hair growth, among which the Golgi-localized PAT24/TIP GROWTH DEFECTIVE1 (TIP1) plays a major role while the tonoplast-localized PAT10 plays a secondary role in root hair growth. A specific inhibitor for protein palmitoylation, 2-bromopalmitate (2-BP), compromised root hair elongation and polarity. Using various probes specific for cellular processes, we demonstrated that 2-BP impaired the dynamic polymerization of actin microfilaments (MF), the asymmetric plasma membrane (PM) localization of phosphatidylinositol (4,5)-bisphosphate (PIP2), the dynamic distribution of RabA4b-positive post-Golgi secretion, and endocytic trafficking in root hairs.
Conclusions: By combining pharmacological and genetic approaches and using root hairs as a model, we show that protein palmitoylation, regulated by protein S-acyl transferases at different endomembrane compartments such as the Golgi and the vacuole, is critical for the polar growth of root hairs in Arabidopsis. Inhibition of protein palmitoylation by 2-BP disturbed key intracellular activities in root hairs. Although some of these effects are likely indirect, the cytological data reported here will contribute to a deep understanding of protein palmitoylation during tip growth in plants.
Figures



Similar articles
-
Arabidopsis PROTEIN S-ACYL TRANSFERASE4 mediates root hair growth.Plant J. 2017 Apr;90(2):249-260. doi: 10.1111/tpj.13484. Epub 2017 Mar 13. Plant J. 2017. PMID: 28107768
-
PLURIPETALA mediates ROP2 localization and stability in parallel to SCN1 but synergistically with TIP1 in root hairs.Plant J. 2016 Jun;86(5):413-25. doi: 10.1111/tpj.13179. Plant J. 2016. PMID: 27037800
-
Protein S-acyl transferase 4 controls nucleus position during root hair tip growth.Plant Signal Behav. 2017 Apr 3;12(4):e1311438. doi: 10.1080/15592324.2017.1311438. Plant Signal Behav. 2017. PMID: 28368733 Free PMC article.
-
Spatial organization of palmitoyl acyl transferases governs substrate localization and function.Mol Membr Biol. 2019 Dec;35(1):60-75. doi: 10.1080/09687688.2019.1710274. Mol Membr Biol. 2019. PMID: 31969037 Free PMC article. Review.
-
Receptor-like protein kinases: Key regulators controlling root hair development in Arabidopsis thaliana.J Integr Plant Biol. 2018 Sep;60(9):841-850. doi: 10.1111/jipb.12663. Epub 2018 Aug 23. J Integr Plant Biol. 2018. PMID: 29727051 Review.
Cited by
-
Arabidopsis VAC14 Is Critical for Pollen Development through Mediating Vacuolar Organization.Plant Physiol. 2018 Aug;177(4):1529-1538. doi: 10.1104/pp.18.00495. Epub 2018 Jun 8. Plant Physiol. 2018. PMID: 29884680 Free PMC article.
-
Small GTPases in plant biotic interactions.Small GTPases. 2019 Sep;10(5):350-360. doi: 10.1080/21541248.2017.1333557. Epub 2017 Jun 23. Small GTPases. 2019. PMID: 28644721 Free PMC article. Review.
-
Vacuolar trafficking in pollen tube growth and guidance.Plant Signal Behav. 2018;13(5):e1464854. doi: 10.1080/15592324.2018.1464854. Epub 2018 Jun 26. Plant Signal Behav. 2018. PMID: 29701540 Free PMC article. Review.
-
Global profiling of protein S-palmitoylation in the second-generation merozoites of Eimeria tenella.Parasitol Res. 2024 Apr 22;123(4):190. doi: 10.1007/s00436-024-08204-2. Parasitol Res. 2024. PMID: 38647704
-
Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction.Int J Mol Sci. 2024 Sep 12;25(18):9845. doi: 10.3390/ijms25189845. Int J Mol Sci. 2024. PMID: 39337338 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

