A nontoxic polypeptide oligomer with a fungicide potency under agricultural conditions which is equal or greater than that of their chemical counterparts
- PMID: 25849076
- PMCID: PMC4388547
- DOI: 10.1371/journal.pone.0122095
A nontoxic polypeptide oligomer with a fungicide potency under agricultural conditions which is equal or greater than that of their chemical counterparts
Abstract
There are literally hundreds of polypeptides described in the literature which exhibit fungicide activity. Tens of them have had attempted protection by patent applications but none, as far as we are aware, have found application under real agricultural conditions. The reasons behind may be multiple where the sensitivity to the Sun UV radiation can come in first place. Here we describe a multifunctional glyco-oligomer with 210 kDa which is mainly composed by a 20 kDa polypeptide termed Blad that has been previously shown to be a stable intermediary product of β-conglutin catabolism. This oligomer accumulates exclusively in the cotyledons of Lupinus species, between days 4 and 12 after the onset of germination. Blad-oligomer reveals a plethora of biochemical properties, like lectin and catalytic activities, which are not unusual per si, but are remarkable when found to coexist in the same protein molecule. With this vast range of chemical characteristics, antifungal activity arises almost as a natural consequence. The biological significance and potential technological applications of Blad-oligomer as a plant fungicide to agriculture, its uniqueness stems from being of polypeptidic in nature, and with efficacies which are either equal or greater than the top fungicides currently in the market are addressed.
Conflict of interest statement
Figures
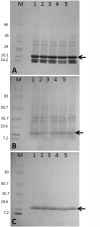
Similar articles
-
Blad-Containing Oligomer Fungicidal Activity on Human Pathogenic Yeasts. From the Outside to the Inside of the Target Cell.Front Microbiol. 2016 Nov 14;7:1803. doi: 10.3389/fmicb.2016.01803. eCollection 2016. Front Microbiol. 2016. PMID: 27933037 Free PMC article.
-
The unique biosynthetic route from lupinus beta-conglutin gene to blad.PLoS One. 2010 Jan 6;5(1):e8542. doi: 10.1371/journal.pone.0008542. PLoS One. 2010. PMID: 20066045 Free PMC article.
-
Evaluation of fungicides enestroburin and SYP1620 on their inhibitory activities to fungi and oomycetes and systemic translocation in plants.Pestic Biochem Physiol. 2014 Jun;112:19-25. doi: 10.1016/j.pestbp.2014.05.004. Epub 2014 Jun 4. Pestic Biochem Physiol. 2014. PMID: 24974113
-
The strobilurin fungicides.Pest Manag Sci. 2002 Jul;58(7):649-62. doi: 10.1002/ps.520. Pest Manag Sci. 2002. PMID: 12146165 Review.
-
Azole fungicides - understanding resistance mechanisms in agricultural fungal pathogens.Pest Manag Sci. 2015 Aug;71(8):1054-8. doi: 10.1002/ps.4029. Epub 2015 May 20. Pest Manag Sci. 2015. PMID: 25914201 Review.
Cited by
-
Protein profiling of water and alkali soluble cottonseed protein isolates.Sci Rep. 2018 Jun 18;8(1):9306. doi: 10.1038/s41598-018-27671-z. Sci Rep. 2018. PMID: 29915326 Free PMC article.
-
Narrow-Leafed Lupin (Lupinus angustifolius) β1- and β6-Conglutin Proteins Exhibit Antifungal Activity, Protecting Plants against Necrotrophic Pathogen Induced Damage from Sclerotinia sclerotiorum and Phytophthora nicotianae.Front Plant Sci. 2016 Dec 9;7:1856. doi: 10.3389/fpls.2016.01856. eCollection 2016. Front Plant Sci. 2016. PMID: 28018392 Free PMC article.
-
Bridging the Gap to Non-toxic Fungal Control: Lupinus-Derived Blad-Containing Oligomer as a Novel Candidate to Combat Human Pathogenic Fungi.Front Microbiol. 2017 Jun 28;8:1182. doi: 10.3389/fmicb.2017.01182. eCollection 2017. Front Microbiol. 2017. PMID: 28702011 Free PMC article.
-
Fungicides and the Grapevine Wood Mycobiome: A Case Study on Tracheomycotic Ascomycete Phaeomoniella chlamydospora Reveals Potential for Two Novel Control Strategies.Front Plant Sci. 2019 Oct 31;10:1405. doi: 10.3389/fpls.2019.01405. eCollection 2019. Front Plant Sci. 2019. PMID: 31737020 Free PMC article.
-
Blad-Containing Oligomer Fungicidal Activity on Human Pathogenic Yeasts. From the Outside to the Inside of the Target Cell.Front Microbiol. 2016 Nov 14;7:1803. doi: 10.3389/fmicb.2016.01803. eCollection 2016. Front Microbiol. 2016. PMID: 27933037 Free PMC article.
References
-
- Ferreira RB, Monteiro S, Freitas R, Santos C, Chen Z, Batista L, et al. (2006) Fungal pathogens: the battle for plant infection. Crit. Rev. Plant Sci. 25: 506–524. 10.1080/07352680601054610 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

