Spectroscopic Characterization of Structural Changes in Membrane Scaffold Proteins Entrapped within Mesoporous Silica Gel Monoliths
- PMID: 25849085
- PMCID: PMC5522711
- DOI: 10.1021/acsami.5b00898
Spectroscopic Characterization of Structural Changes in Membrane Scaffold Proteins Entrapped within Mesoporous Silica Gel Monoliths
Abstract
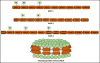
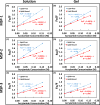
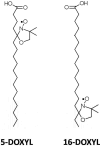
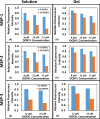
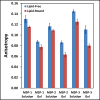
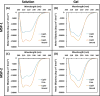
The changes in the orientation and conformation of three different membrane scaffold proteins (MSPs) upon entrapment in sol-gel-derived mesoporous silica monoliths were investigated. MSPs were examined in either a lipid-free or a lipid-bound conformation, where the proteins were associated with lipids to form nanolipoprotein particles (NLPs). NLPs are water-soluble, disk-shaped patches of a lipid bilayer that have amphiphilic MSPs shielding the hydrophobic lipid tails. The NLPs in this work had an average thickness of 5 nm and diameters of 9.2, 9.7, and 14.8 nm. We have previously demonstrated that NLPs are more suitable lipid-based structures for silica gel entrapment than liposomes because of their size compatibility with the mesoporous network (2-50 nm) and minimally altered structure after encapsulation. Here we further elaborate on that work by using a variety of spectroscopic techniques to elucidate whether or not different MSPs maintain their protein-lipid interactions after encapsulation. Fluorescence spectroscopy and quenching of the tryptophan residues with acrylamide, 5-DOXYL-stearic acid, and 16-DOXYL-stearic acid were used to determine the MSP orientation. We also utilized fluorescence anisotropy of tryptophans to measure the relative size of the NLPs and MSP aggregates after entrapment. Finally, circular dichroism spectroscopy was used to examine the secondary structure of the MSPs. Our results showed that, after entrapment, all of the lipid-bound MSPs maintained orientations that were minimally changed and indicative of association with lipids in NLPs. The tryptophan residues appeared to remain buried within the hydrophobic core of the lipid tails in the NLPs and appropriately spaced from the bilayer center. Also, after entrapment, lipid-bound MSPs maintained a high degree of α-helical content, a secondary structure associated with protein-lipid interactions. These findings demonstrate that NLPs are capable of serving as viable hosts for functional integral membrane proteins in the synthesis of sol-gel-derived bioinorganic hybrid nanomaterials.
Keywords: biohybrid material; membrane scaffold protein; mesoporous silica; nanolipoprotein particle; protein−lipid interactions; tryptophan fluorescence.
Figures
Similar articles
-
Analysis of lipid phase behavior and protein conformational changes in nanolipoprotein particles upon entrapment in sol-gel-derived silica.Langmuir. 2014 Aug 19;30(32):9780-8. doi: 10.1021/la5025058. Epub 2014 Aug 6. Langmuir. 2014. PMID: 25062385 Free PMC article.
-
Amphipathic alpha-helix bundle organization of lipid-free chicken apolipoprotein A-I.Biochemistry. 1999 Apr 6;38(14):4327-34. doi: 10.1021/bi982597p. Biochemistry. 1999. PMID: 10194351
-
Lipid and Protein Transfer between Nanolipoprotein Particles and Supported Lipid Bilayers.Langmuir. 2019 Sep 17;35(37):12071-12078. doi: 10.1021/acs.langmuir.9b01288. Epub 2019 Sep 6. Langmuir. 2019. PMID: 31442053 Free PMC article.
-
Nonmicellar systems for solution NMR spectroscopy of membrane proteins.Curr Opin Struct Biol. 2010 Aug;20(4):471-9. doi: 10.1016/j.sbi.2010.05.006. Curr Opin Struct Biol. 2010. PMID: 20570504 Free PMC article. Review.
-
Cell-Free Co-Translational Approaches for Producing Mammalian Receptors: Expanding the Cell-Free Expression Toolbox Using Nanolipoproteins.Front Pharmacol. 2019 Jul 3;10:744. doi: 10.3389/fphar.2019.00744. eCollection 2019. Front Pharmacol. 2019. PMID: 31333463 Free PMC article. Review.
Cited by
-
Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications.J Nanobiotechnology. 2022 Mar 12;20(1):126. doi: 10.1186/s12951-022-01315-x. J Nanobiotechnology. 2022. PMID: 35279150 Free PMC article. Review.
-
Dynamics of Crowding-Induced Mixing in Phase Separated Lipid Bilayers.J Phys Chem B. 2016 Nov 3;120(43):11180-11190. doi: 10.1021/acs.jpcb.6b07119. Epub 2016 Oct 25. J Phys Chem B. 2016. PMID: 27723342 Free PMC article.
-
Characterization of ApoJ-reconstituted high-density lipoprotein (rHDL) nanodisc for the potential treatment of cerebral β-amyloidosis.Sci Rep. 2017 Nov 7;7(1):14637. doi: 10.1038/s41598-017-15215-w. Sci Rep. 2017. PMID: 29116115 Free PMC article.
-
Crowding-Induced Mixing Behavior of Lipid Bilayers: Examination of Mixing Energy, Phase, Packing Geometry, and Reversibility.Langmuir. 2016 May 10;32(18):4688-97. doi: 10.1021/acs.langmuir.6b00831. Epub 2016 Apr 26. Langmuir. 2016. PMID: 27096947 Free PMC article.
-
Intrinsic Fluorescence of PAMAM Dendrimers-Quenching Studies.Polymers (Basel). 2018 May 17;10(5):540. doi: 10.3390/polym10050540. Polymers (Basel). 2018. PMID: 30966574 Free PMC article.
References
-
- Ruiz-Hitzky E, Ariga K, Lvov Y. Bio-Inorganic Hybrid Nanomaterials: Strategies, Syntheses, Characterization and Applications. WILEY-VCH; Weinheim, Germany: 2008.
-
- Avnir D, Coradin T, Lev O, Livage J. Recent Bio-Applications of Sol-Gel Materials. J Mater Chem. 2006;16:1013.
-
- Schlipf DM, Rankin SE, Knutson BL. Pore-Size Dependent Protein Adsorption and Protection from Proteolytic Hydrolysis in Tailored Mesoporous Silica Particles. ACS Appl. Mater. Interfaces. 2013;5:10111–10117. - PubMed
-
- Nassif N, Livage J. From Diatoms to Silica-Based Biohybrids. Chem Soc Rev. 2011;40:849–59. - PubMed
-
- Kato M, Sakai-Kato K, Toyo’oka T. Silica Sol-Gel Monolithic Materials and Their Use in a Variety of Applications. J. Sep. Sci. 2005;28:1893–1908.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

