Recombinant hamster oviductin is biologically active and exerts positive effects on sperm functions and sperm-oocyte binding
- PMID: 25849110
- PMCID: PMC4388664
- DOI: 10.1371/journal.pone.0123003
Recombinant hamster oviductin is biologically active and exerts positive effects on sperm functions and sperm-oocyte binding
Abstract
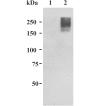
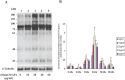
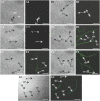
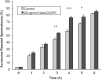
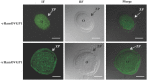
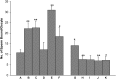
Studies carried out in several mammalian species suggest that oviductin, also known as oviduct-specific glycoprotein or OVGP1, plays a key role in sperm capacitation, fertilization, and development of early embryos. In the present study, we used recombinant DNA technology to produce, for the first time, recombinant hamster OVGP1 (rHamOVGP1) in human embryonic kidney 293 (HEK293) cells. rHamOVGP1 secreted in the culture medium was purified by affinity chromatography. The resulting protein migrated as a poly-dispersed band of 160-350 kDa on SDS-PAGE corresponding to the molecular mass of the native HamOVGP1. Subsequent mass spectrometric analysis of the purified rHamOVGP1 confirmed its identity as HamOVGP1. Immunocytochemistry demonstrated binding of rHamOVGP1 to the mid-piece and head of hamster sperm and to the zona pellucida (ZP) of ovarian oocytes. In vitro functional experiments showed that addition of rHamOVGP1 in the capacitation medium further enhanced tyrosine phosphorylation of two sperm proteins of approximately 75 kDa and 83 kDa in a time-dependent manner. After 3 hours of incubation in the presence of rHamOVGP1, a significant increase in acrosome reaction was measured. Pretreatment of either sperm or oocyte with 20 μg/ml of rHamOVGP1 prior to sperm-egg binding assay significantly increased the number of sperm bound to the ZP. Addition of rHamOVGP1 in the medium during sperm-egg binding with either oocyte or sperm pretreated with rHamOVGP1 also saw an increase in the number of sperm bound to ZP. In all experimental conditions, the effect of rHamOVGP1 on sperm-oocyte binding was negated by the addition of monoclonal anti-HamOVGP1 antibody. The successful production and purification of a biologically active rHamOVGP1 will allow further exploration of the function of this glycoprotein in reproductive function.
Conflict of interest statement
Figures




Similar articles
-
Human OVGP1 enhances tyrosine phosphorylation of proteins in the fibrous sheath involving AKAP3 and increases sperm-zona binding.J Assist Reprod Genet. 2019 Jul;36(7):1363-1377. doi: 10.1007/s10815-019-01502-0. Epub 2019 Jun 28. J Assist Reprod Genet. 2019. PMID: 31254143 Free PMC article.
-
Surface mapping of binding of oviductin to the plasma membrane of golden hamster spermatozoa during in vitro capacitation and acrosome reaction.Mol Reprod Dev. 2006 Jun;73(6):756-66. doi: 10.1002/mrd.20459. Mol Reprod Dev. 2006. PMID: 16493683
-
Oviductin sets the species-specificity of the mammalian zona pellucida.Elife. 2025 Jun 9;13:RP101338. doi: 10.7554/eLife.101338. Elife. 2025. PMID: 40488743 Free PMC article.
-
[Elucidation of the mechanism of fertilization and clinical application of assisted reproductive technology].Nihon Sanka Fujinka Gakkai Zasshi. 1996 Aug;48(8):578-90. Nihon Sanka Fujinka Gakkai Zasshi. 1996. PMID: 8808826 Review. Japanese.
-
Dynamic regulation of sperm interactions with the zona pellucida prior to and after fertilisation.Reprod Fertil Dev. 2012;25(1):26-37. doi: 10.1071/RD12277. Reprod Fertil Dev. 2012. PMID: 23244826 Review.
Cited by
-
The sperm-interacting proteome in the bovine isthmus and ampulla during the periovulatory period.J Anim Sci Biotechnol. 2023 Feb 17;14(1):30. doi: 10.1186/s40104-022-00811-2. J Anim Sci Biotechnol. 2023. PMID: 36797800 Free PMC article.
-
Molecular Evolution of the Ovgp1 Gene in the Subfamily Murinae.Animals (Basel). 2024 Dec 29;15(1):55. doi: 10.3390/ani15010055. Animals (Basel). 2024. PMID: 39794998 Free PMC article.
-
Effect of recombinant and native buffalo OVGP1 on sperm functions and in vitro embryo development: a comparative study.J Anim Sci Biotechnol. 2017 Sep 1;8:69. doi: 10.1186/s40104-017-0201-5. eCollection 2017. J Anim Sci Biotechnol. 2017. PMID: 28883914 Free PMC article.
-
Pre-Treatment of Swine Oviductal Epithelial Cells with Progesterone Increases the Sperm Fertilizing Ability in an IVF Model.Animals (Basel). 2022 May 6;12(9):1191. doi: 10.3390/ani12091191. Animals (Basel). 2022. PMID: 35565617 Free PMC article.
-
Spatiotemporal profiling of the bovine oviduct fluid proteome around the time of ovulation.Sci Rep. 2022 Mar 9;12(1):4135. doi: 10.1038/s41598-022-07929-3. Sci Rep. 2022. PMID: 35264682 Free PMC article.
References
-
- Buhi WC, Alvarez IM, Kouba AJ. Secreted proteins of the oviduct. Cells Tissues Organs. 2000; 166: 165–179. - PubMed
-
- Leese HJ. The formation and function of oviduct fluid. J Reprod Fertil. 1988; 82: 843–856. - PubMed
-
- Kapur RP, Johnson LV. Ultrastructural evidence that specialized regions of the murine oviduct contribute a glycoprotein to the extracellular matrix of mouse oocytes. Anat Rec. 1988; 221: 720–729. - PubMed
-
- Sendai Y, Komiya H, Suzuki K, Onuma T, Kikuchi M, Hoshi H, et al. Molecular cloning and characterization of a mouse oviduct-specific glycoprotein. Biol Reprod. 1995; 53: 285–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

