δ-tocotrienol induces human bladder cancer cell growth arrest, apoptosis and chemosensitization through inhibition of STAT3 pathway
- PMID: 25849286
- PMCID: PMC4388509
- DOI: 10.1371/journal.pone.0122712
δ-tocotrienol induces human bladder cancer cell growth arrest, apoptosis and chemosensitization through inhibition of STAT3 pathway
Abstract
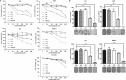
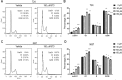
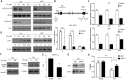
Vitamin E intake has been implicated in reduction of bladder cancer risk. However, the mechanisms remain elusive. Here we reported that δ-tocotrienol (δ-T3), one of vitamin E isomers, possessed the most potent cytotoxic capacity against human bladder cancer cells, compared with other Vitamin E isomers. δ-T3 inhibited cancer cell proliferation and colonogenicity through induction of G1 phase arrest and apoptosis. Western blotting assay revealed that δ-T3 increased the expression levels of cell cycle inhibitors (p21, p27), pro-apoptotic protein (Bax) and suppressed expression levels of cell cycle protein (Cyclin D1), anti-apoptotic proteins (Bcl-2, Bcl-xL and Mcl-1), resulting in the Caspase-3 activation and cleavage of PARP. Moreover, the δ-T3 treatment inhibited ETK phosphorylation level and induced SHP-1 expression, which was correlated with downregulation of STAT3 activation. In line with this, δ-T3 reduced the STAT3 protein level in nuclear fraction, as well as its transcription activity. Knockdown of SHP-1 partially reversed δ-T3-induced cell growth arrest. Importantly, low dose of δ-T3 sensitized Gemcitabine-induced cytotoxic effects on human bladder cancer cells. Overall, our findings demonstrated, for the first time, the cytotoxic effects of δ-T3 on bladder cancer cells and suggest that δ-T3 might be a promising chemosensitization reagent for Gemcitabine in bladder cancer treatment.
Conflict of interest statement
Figures



Similar articles
-
γ-Tocotrienol but not γ-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents.J Biol Chem. 2010 Oct 22;285(43):33520-33529. doi: 10.1074/jbc.M110.158378. Epub 2010 Aug 18. J Biol Chem. 2010. Retraction in: J Biol Chem. 2016 Aug 5;291(32):16922. doi: 10.1074/jbc.A110.158378. PMID: 20720018 Free PMC article. Retracted.
-
γ-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent.Br J Pharmacol. 2011 May;163(2):283-98. doi: 10.1111/j.1476-5381.2010.01187.x. Br J Pharmacol. 2011. PMID: 21198544 Free PMC article.
-
Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway.Biomolecules. 2020 Apr 9;10(4):577. doi: 10.3390/biom10040577. Biomolecules. 2020. PMID: 32283796 Free PMC article.
-
δ-Tocotrienol treatment is more effective against hypoxic tumor cells than normoxic cells: potential implications for cancer therapy.J Nutr Biochem. 2015 Aug;26(8):832-40. doi: 10.1016/j.jnutbio.2015.02.011. Epub 2015 Apr 14. J Nutr Biochem. 2015. PMID: 25979648
-
Pesticides and Bladder Cancer: Mechanisms Leading to Anti-Cancer Drug Chemoresistance and New Chemosensitization Strategies.Int J Mol Sci. 2023 Jul 13;24(14):11395. doi: 10.3390/ijms241411395. Int J Mol Sci. 2023. PMID: 37511154 Free PMC article. Review.
Cited by
-
Musashi-2 Silencing Exerts Potent Activity against Acute Myeloid Leukemia and Enhances Chemosensitivity to Daunorubicin.PLoS One. 2015 Aug 26;10(8):e0136484. doi: 10.1371/journal.pone.0136484. eCollection 2015. PLoS One. 2015. PMID: 26308531 Free PMC article.
-
Molecular Mechanisms of Action of Tocotrienols in Cancer: Recent Trends and Advancements.Int J Mol Sci. 2019 Feb 2;20(3):656. doi: 10.3390/ijms20030656. Int J Mol Sci. 2019. PMID: 30717416 Free PMC article. Review.
-
δ-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis.Oncotarget. 2017 May 9;8(19):31554-31567. doi: 10.18632/oncotarget.15767. Oncotarget. 2017. PMID: 28404939 Free PMC article.
-
Tocotrienols Modulate a Life or Death Decision in Cancers.Int J Mol Sci. 2019 Jan 16;20(2):372. doi: 10.3390/ijms20020372. Int J Mol Sci. 2019. PMID: 30654580 Free PMC article. Review.
-
Combination of gemcitabine and erlotinib inhibits recurrent pancreatic cancer growth in mice via the JAK-STAT pathway.Oncol Rep. 2018 Mar;39(3):1081-1089. doi: 10.3892/or.2018.6198. Epub 2018 Jan 8. Oncol Rep. 2018. PMID: 29328487 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

