Tissue factor-expressing tumor cells can bind to immobilized recombinant tissue factor pathway inhibitor under static and shear conditions in vitro
- PMID: 25849335
- PMCID: PMC4388665
- DOI: 10.1371/journal.pone.0123717
Tissue factor-expressing tumor cells can bind to immobilized recombinant tissue factor pathway inhibitor under static and shear conditions in vitro
Abstract
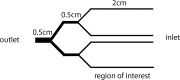
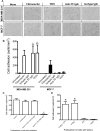
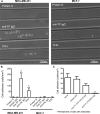
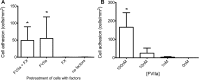
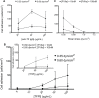
Mammary tumors and malignant breast cancer cell lines over-express the coagulation factor, tissue factor (TF). High expression of TF is associated with a poor prognosis in breast cancer. Tissue factor pathway inhibitor (TFPI), the endogenous inhibitor of TF, is constitutively expressed on the endothelium. We hypothesized that TF-expressing tumor cells can bind to immobilized recombinant TFPI, leading to arrest of the tumor cells under shear in vitro. We evaluated the adhesion of breast cancer cells to immobilized TFPI under static and shear conditions (0.35 - 1.3 dyn/cm2). We found that high-TF-expressing breast cancer cells, MDA-MB-231 (with a TF density of 460,000/cell), but not low TF-expressing MCF-7 (with a TF density of 1,400/cell), adhered to recombinant TFPI, under static and shear conditions. Adhesion of MDA-MB-231 cells to TFPI required activated factor VII (FVIIa), but not FX, and was inhibited by a factor VIIa-blocking anti-TF antibody. Under shear, adhesion to TFPI was dependent on the TFPI-coating concentration, FVIIa concentration and shear stress, with no observed adhesion at shear stresses greater than 1.0 dyn/cm2. This is the first study showing that TF-expressing tumor cells can be captured by immobilized TFPI, a ligand constitutively expressed on the endothelium, under low shear in vitro. Based on our results, we hypothesize that TFPI could be a novel ligand mediating the arrest of TF-expressing tumor cells in high TFPI-expressing vessels under conditions of low shear during metastasis.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

