Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis
- PMID: 25849352
- PMCID: PMC4388826
- DOI: 10.1371/journal.pmed.1001810
Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis
Erratum in
-
Correction: Geographic and Temporal Trends in the Molecular Epidemiology and Genetic Mechanisms of Transmitted HIV-1 Drug Resistance: An Individual-Patient- and Sequence-Level Meta-Analysis.PLoS Med. 2015 Jun 1;12(6):e1001845. doi: 10.1371/journal.pmed.1001845. eCollection 2015 Jun. PLoS Med. 2015. PMID: 26030872 Free PMC article. No abstract available.
Abstract
Background: Regional and subtype-specific mutational patterns of HIV-1 transmitted drug resistance (TDR) are essential for informing first-line antiretroviral (ARV) therapy guidelines and designing diagnostic assays for use in regions where standard genotypic resistance testing is not affordable. We sought to understand the molecular epidemiology of TDR and to identify the HIV-1 drug-resistance mutations responsible for TDR in different regions and virus subtypes.
Methods and findings: We reviewed all GenBank submissions of HIV-1 reverse transcriptase sequences with or without protease and identified 287 studies published between March 1, 2000, and December 31, 2013, with more than 25 recently or chronically infected ARV-naïve individuals. These studies comprised 50,870 individuals from 111 countries. Each set of study sequences was analyzed for phylogenetic clustering and the presence of 93 surveillance drug-resistance mutations (SDRMs). The median overall TDR prevalence in sub-Saharan Africa (SSA), south/southeast Asia (SSEA), upper-income Asian countries, Latin America/Caribbean, Europe, and North America was 2.8%, 2.9%, 5.6%, 7.6%, 9.4%, and 11.5%, respectively. In SSA, there was a yearly 1.09-fold (95% CI: 1.05-1.14) increase in odds of TDR since national ARV scale-up attributable to an increase in non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance. The odds of NNRTI-associated TDR also increased in Latin America/Caribbean (odds ratio [OR] = 1.16; 95% CI: 1.06-1.25), North America (OR = 1.19; 95% CI: 1.12-1.26), Europe (OR = 1.07; 95% CI: 1.01-1.13), and upper-income Asian countries (OR = 1.33; 95% CI: 1.12-1.55). In SSEA, there was no significant change in the odds of TDR since national ARV scale-up (OR = 0.97; 95% CI: 0.92-1.02). An analysis limited to sequences with mixtures at less than 0.5% of their nucleotide positions—a proxy for recent infection—yielded trends comparable to those obtained using the complete dataset. Four NNRTI SDRMs—K101E, K103N, Y181C, and G190A—accounted for >80% of NNRTI-associated TDR in all regions and subtypes. Sixteen nucleoside reverse transcriptase inhibitor (NRTI) SDRMs accounted for >69% of NRTI-associated TDR in all regions and subtypes. In SSA and SSEA, 89% of NNRTI SDRMs were associated with high-level resistance to nevirapine or efavirenz, whereas only 27% of NRTI SDRMs were associated with high-level resistance to zidovudine, lamivudine, tenofovir, or abacavir. Of 763 viruses with TDR in SSA and SSEA, 725 (95%) were genetically dissimilar; 38 (5%) formed 19 sequence pairs. Inherent limitations of this study are that some cohorts may not represent the broader regional population and that studies were heterogeneous with respect to duration of infection prior to sampling.
Conclusions: Most TDR strains in SSA and SSEA arose independently, suggesting that ARV regimens with a high genetic barrier to resistance combined with improved patient adherence may mitigate TDR increases by reducing the generation of new ARV-resistant strains. A small number of NNRTI-resistance mutations were responsible for most cases of high-level resistance, suggesting that inexpensive point-mutation assays to detect these mutations may be useful for pre-therapy screening in regions with high levels of TDR. In the context of a public health approach to ARV therapy, a reliable point-of-care genotypic resistance test could identify which patients should receive standard first-line therapy and which should receive a protease-inhibitor-containing regimen.
Conflict of interest statement
JHK and MR are employees of the Walter Reed Army Institute of Research, however, the views expressed herein are those of the authors and do not represent the official views of the Departments of the Army or Defense. DD has received honoraria and travel grants from Viiv Healthcare, Janssen-Cilag, Gilead-Sciences, MSD and BMS for participation to advisory boards and international conferences. SHE collaborates on research studies with investigators from Abbott Laboratories (distributor of the ViroSeq HIV-1 Genotyping System). Abbott Laboratories has provided reagents and performed testing for some collaborative studies. PF has received paid employment for educational presentation (Bristol-Myers Squibb, Janssen-Cilag), travel grants and honoraria for speaking or participation at meetings (Bristol-Myers Squibb, MSD, Gilead, Astellas). WS has received honoraria for speaking from Viiv, MSD, Janssen and Torii. PRH has received grants from, served as an ad hoc advisor to, or spoke at various events sponsored by: Pfizer, Glaxo-Smith Kline, Abbott, Merck, Tobira Therapeutics, Virco and Quest Diagnostics. MAP was supported in part from the United States Agency for International Development (USAID), however, the contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. SB is a staff member of the World Health Organization and the contents are the responsibility of the authors and do not necessarily reflect the views of the World Health Organization. JPAI is a member of the Editorial Board of
Figures
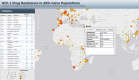
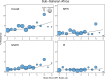




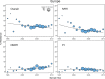



References
-
- Joint United Nations Programme on HIV/AIDS (2013) Global report: UNAIDS report on the global AIDS epidemic 2013. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/.... Accessed 1 March 2015.
-
- Eaton JW, Johnson LF, Salomon JA, Bärnighausen T, Bendavid E, et al. (2012) HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 9: e1001245 10.1371/journal.pmed.1001245 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

