Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx
- PMID: 25849380
- PMCID: PMC4340344
- DOI: 10.1186/s12933-015-0183-6
Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx
Abstract
Background: The prevalence of obesity has dramatically increased worldwide and has attracted rising attention, but the mechanism is still unclear. Previous studies revealed that transient receptor potential vanilloid 1 (TRPV1) channels take part in weight loss by enhancing intracellular Ca2+ levels. However, the potential mechanism of the effect of dietary capsaicin on obesity is not completely understood. Ca2+ transfer induced by connexin43 (Cx43) molecules between coupled cells takes part in adipocyte differentiation. Whether TRPV1-evoked alterations in Cx43-mediated adipocyte-to-adipocyte communication play a role in obesity is unknown.
Materials and methods: We investigated whether Cx43 participated in TRPV1-mediated adipocyte lipolysis in cultured 3T3-L1 preadipocytes and visceral adipose tissues from humans and wild-type (WT) and TRPV1-deficient (TRPV1-/-) mice.
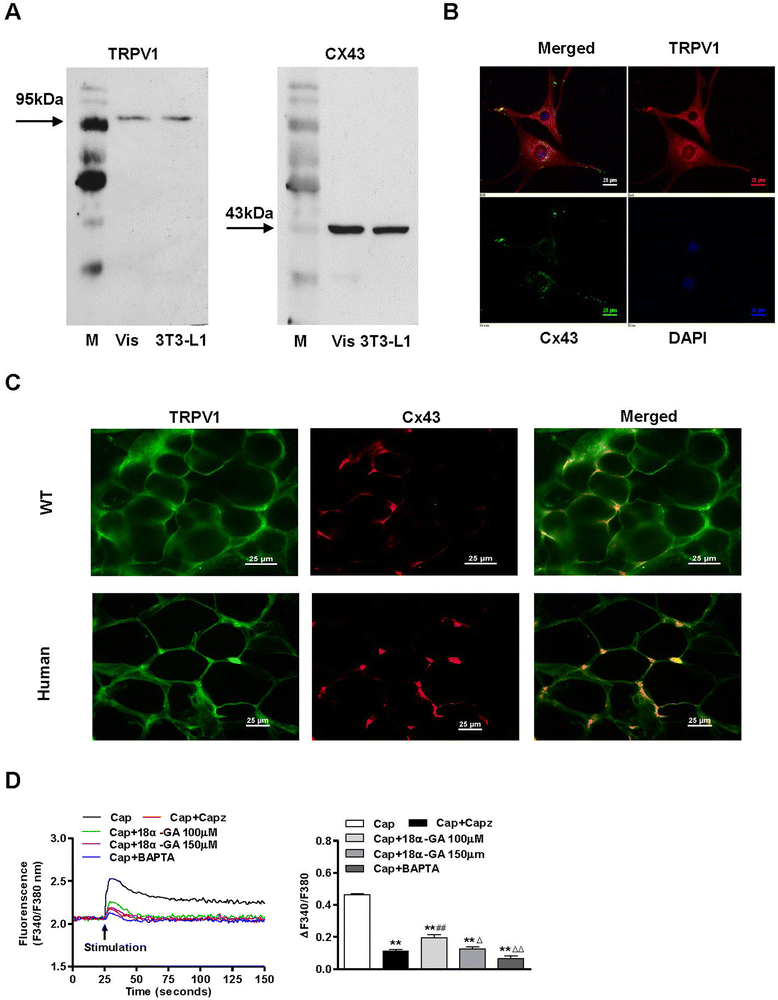
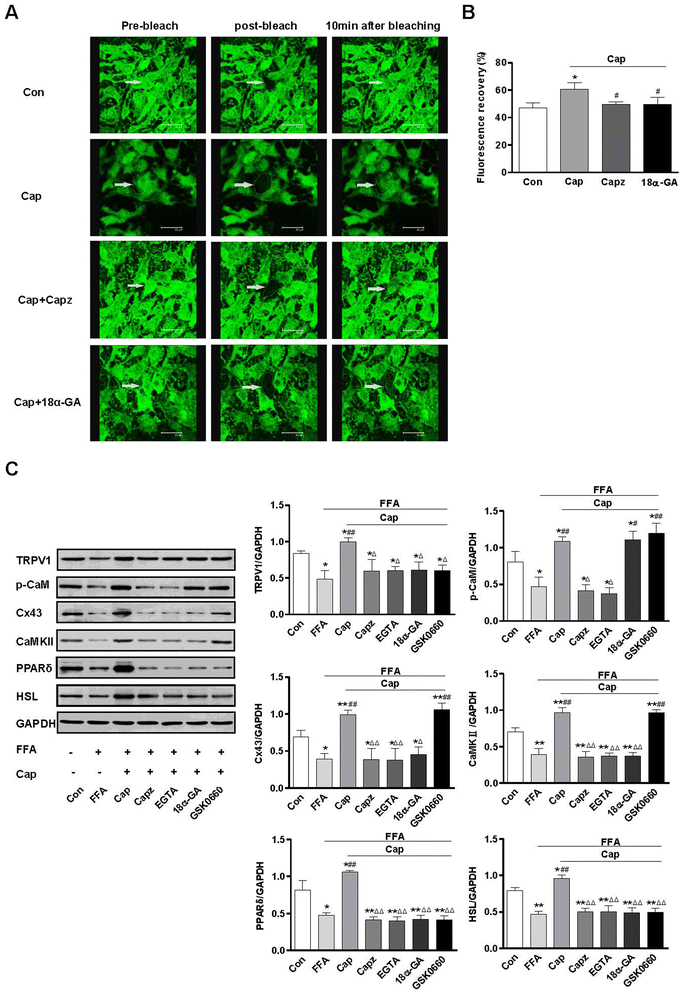
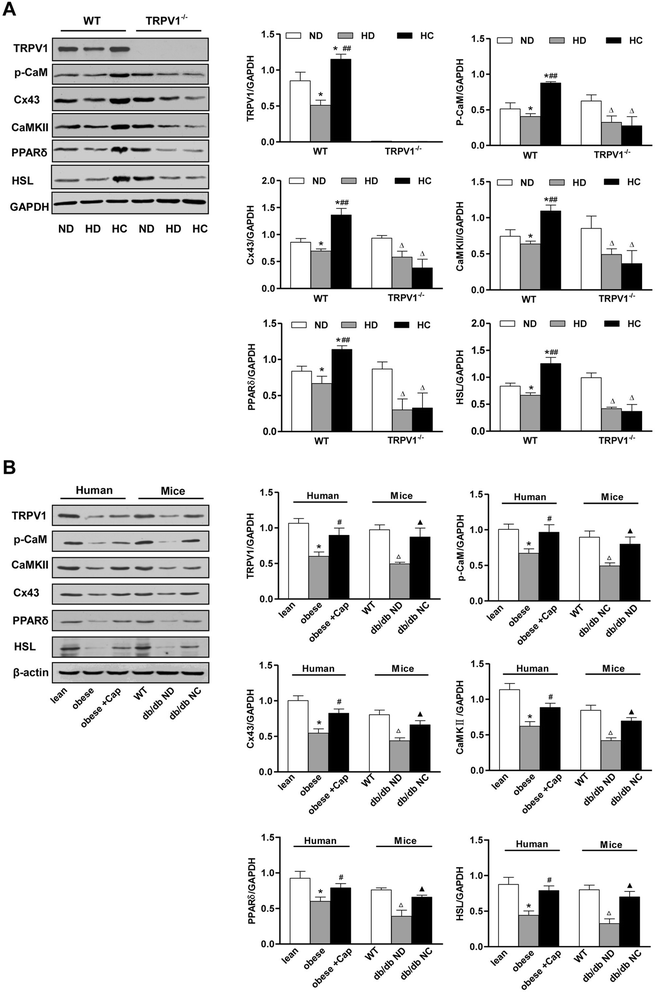
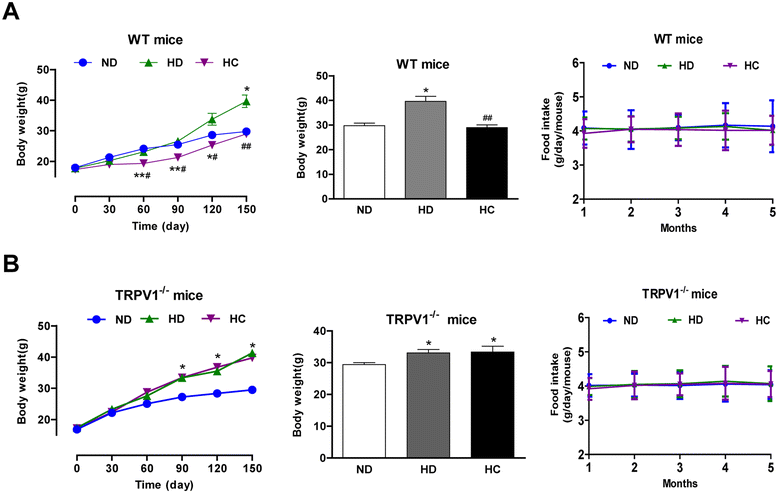
Results: TRPV1 and Cx43 co-expressed in mesenteric adipose tissue. TRPV1 activation by capsaicin increased the influx of Ca2+ in 3T3-L1 preadipocytes and promoted cell lipolysis, as shown by Oil-red O staining. These effects were deficient when capsazepine, a TRPV1 antagonist, and 18 alpha-glycyrrhetinic acid (18α-GA), a gap-junction inhibitor, were administered. Long-term chronic dietary capsaicin reduced the weights of perirenal, mesenteric and testicular adipose tissues in WT mice fed a high-fat diet. Capsaicin increased the expression levels of p-CaM, Cx43, CaMKII, PPARδ and HSL in mesenteric adipose tissues from WT mice fed a high-fat diet, db/db mice, as well as obese humans, but these effects of capsaicin were absent in TRPV1-/- mice. Long-term chronic dietary capsaicin decreased the body weights and serum lipids of WT mice, but not TRPV1-/- mice, fed a high-fat diet.
Conclusion: This study demonstrated that capsaicin activation of TRPV1-evoked increased Ca2+ influx in Cx43-mediated adipocyte-to-adipocyte communication promotes lipolysis in both vitro and vivo. TRPV1 activation by dietary capsaicin improves visceral fat remodeling through the up-regulation of Cx43.
Figures

References
-
- de Souza RJ, Bray GA, Carey VJ, Hall KD, LeBoff MS, Loria CM, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am J Clin Nutr. 2012;95(3):614–25. doi: 10.3945/ajcn.111.026328. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

