Structural basis of the substrate specificity of the FPOD/FAOD family revealed by fructosyl peptide oxidase from Eupenicillium terrenum
- PMID: 25849495
- PMCID: PMC4388169
- DOI: 10.1107/S2053230X15003921
Structural basis of the substrate specificity of the FPOD/FAOD family revealed by fructosyl peptide oxidase from Eupenicillium terrenum
Abstract
The FAOD/FPOD family of proteins has the potential to be useful for the longterm detection of blood glucose levels in diabetes patients. A bottleneck for this application is to find or engineer a FAOD/FPOD family enzyme that is specifically active towards α-fructosyl peptides but is inactive towards other types of glycated peptides. Here, the crystal structure of fructosyl peptide oxidase from Eupenicillium terrenum (EtFPOX) is reported at 1.9 Å resolution. In contrast to the previously reported structure of amadoriase II, EtFPOX has an open substrate entrance to accommodate the large peptide substrate. The functions of residues critical for substrate selection are discussed based on structure comparison and sequence alignment. This study reveals the first structural details of group I FPODs that prefer α-fructosyl substrates and could provide significant useful information for uncovering the mechanism of substrate specificity of FAOD/FPODs and guidance towards future enzyme engineering for diagnostic purposes.
Keywords: Eupenicillium terrenum; FAOD/FPOD family; fructosyl peptide oxidase.
Figures



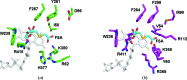
Similar articles
-
Engineering an efficient mutant of Eupenicillium terrenum fructosyl peptide oxidase for the specific determination of hemoglobin A1c.Appl Microbiol Biotechnol. 2019 Feb;103(4):1725-1735. doi: 10.1007/s00253-018-9529-9. Epub 2019 Jan 3. Appl Microbiol Biotechnol. 2019. PMID: 30607487
-
Expression, purification, crystallization and preliminary X-ray diffraction analysis of EtFPOX from Eupenicillium terrenum sp.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013 Jun;69(Pt 6):666-8. doi: 10.1107/S1744309113012128. Epub 2013 May 25. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013. PMID: 23722849 Free PMC article.
-
In silico characterization of fructosyl peptide oxidase properties from Eupenicillium terrenum.J Mol Recognit. 2022 Nov;35(11):e2980. doi: 10.1002/jmr.2980. Epub 2022 Aug 6. J Mol Recognit. 2022. PMID: 35657361
-
Review of fructosyl amino acid oxidase engineering research: a glimpse into the future of hemoglobin A1c biosensing.J Diabetes Sci Technol. 2009 May 1;3(3):585-92. doi: 10.1177/193229680900300324. J Diabetes Sci Technol. 2009. PMID: 20144298 Free PMC article. Review.
-
Enzymatic deglycation of proteins.Arch Biochem Biophys. 2003 Nov 1;419(1):16-24. doi: 10.1016/j.abb.2003.08.011. Arch Biochem Biophys. 2003. PMID: 14568004 Review.
Cited by
-
In Silico Engineering of Enzyme Access Tunnels.Methods Mol Biol. 2022;2397:203-225. doi: 10.1007/978-1-0716-1826-4_11. Methods Mol Biol. 2022. PMID: 34813066
-
X-ray structures of fructosyl peptide oxidases revealing residues responsible for gating oxygen access in the oxidative half reaction.Sci Rep. 2017 Jun 5;7(1):2790. doi: 10.1038/s41598-017-02657-5. Sci Rep. 2017. PMID: 28584265 Free PMC article.
-
Tailoring FPOX enzymes for enhanced stability and expanded substrate recognition.Sci Rep. 2023 Oct 30;13(1):18610. doi: 10.1038/s41598-023-45428-1. Sci Rep. 2023. PMID: 37903872 Free PMC article.
-
Creation of haemoglobin A1c direct oxidase from fructosyl peptide oxidase by combined structure-based site specific mutagenesis and random mutagenesis.Sci Rep. 2019 Jan 30;9(1):942. doi: 10.1038/s41598-018-37806-x. Sci Rep. 2019. PMID: 30700768 Free PMC article.
-
Oxidative cyclization of N-methyl-dopa by a fungal flavoenzyme of the amine oxidase family.J Biol Chem. 2018 Nov 2;293(44):17021-17032. doi: 10.1074/jbc.RA118.004227. Epub 2018 Sep 7. J Biol Chem. 2018. PMID: 30194285 Free PMC article.
References
-
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221. - PubMed
-
- DeLano, W. L. (2002). PyMOL. http://www.pymol.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

