Expression and characterization of protein disulfide isomerase family proteins in bread wheat
- PMID: 25849633
- PMCID: PMC4355359
- DOI: 10.1186/s12870-015-0460-2
Expression and characterization of protein disulfide isomerase family proteins in bread wheat
Abstract
Background: The major wheat seed proteins are storage proteins that are synthesized in the rough endoplasmic reticulum (ER) of starchy endosperm cells. Many of these proteins have intra- and intermolecular disulfide bonds. In eukaryotes, the formation of most intramolecular disulfide bonds in the ER is thought to be catalyzed by protein disulfide isomerase (PDI) family proteins. The cDNAs that encode eight groups of bread wheat (Triticum aestivum L.) PDI family proteins have been cloned, and their expression levels in developing wheat grains have been determined. The purpose of the present study was to characterize the enzymatic properties of the wheat PDI family proteins and clarify their expression patterns in wheat caryopses.
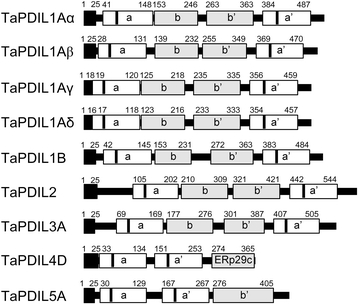
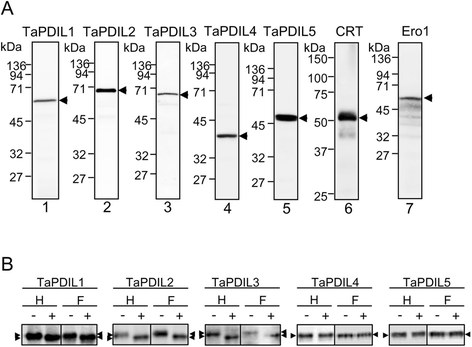
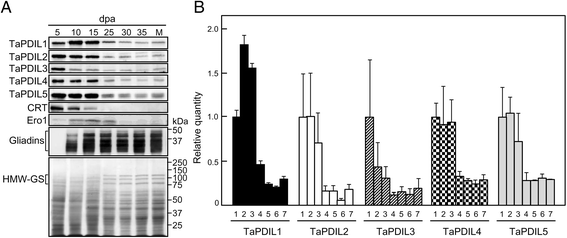
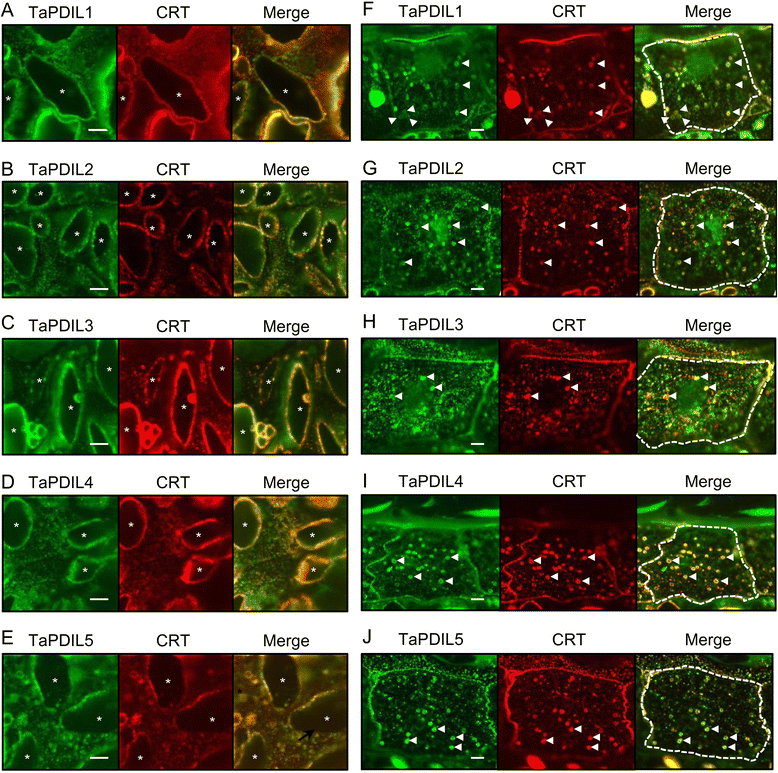
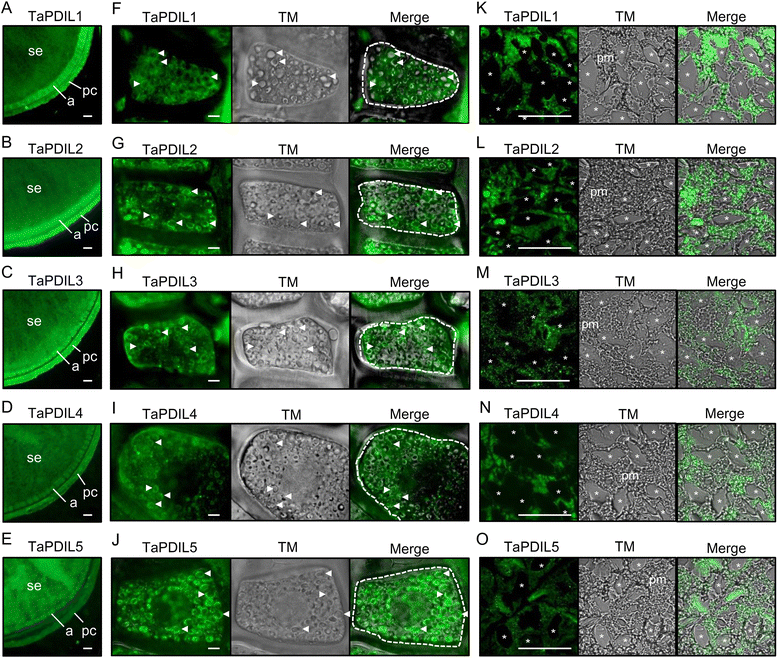
Results: PDI family cDNAs, which are categorized into group I (TaPDIL1Aα, TaPDIL1Aβ, TaPDIL1Aγ, TaPDIL1Aδ, and TaPDIL1B), group II (TaPDIL2), group III (TaPDIL3A), group IV (TaPDIL4D), and group V (TaPDIL5A), were cloned. The expression levels of recombinant TaPDIL1Aα, TaPDIL1B, TaPDIL2, TaPDIL3A, TaPDIL4D, and TaPDIL5A in Escherichia coli were established from the cloned cDNAs. All recombinant proteins were expressed in soluble forms and purified. Aside from TaPDIL3A, the recombinant proteins exhibited oxidative refolding activity on reduced and denatured ribonuclease A. Five groups of PDI family proteins were distributed throughout wheat caryopses, and expression levels of these proteins were higher during grain filling than in the late stage of maturing. Localization of these proteins in the ER was confirmed by fluorescent immunostaining of the immature caryopses. In mature grains, the five groups of PDI family proteins remained in the aleurone cells and the protein matrix of the starchy endosperm.
Conclusions: High expression of PDI family proteins during grain filling in the starchy endosperm suggest that these proteins play an important role in forming intramolecular disulfide bonds in seed storage proteins. In addition, these PDI family proteins that remain in the aleurone layers of mature grains likely assist in folding newly synthesized hydrolytic enzymes during germination.
Figures



Similar articles
-
Purification, characterization, and intracellular localization of glycosylated protein disulfide isomerase from wheat grains.Plant Physiol. 1995 May;108(1):327-35. doi: 10.1104/pp.108.1.327. Plant Physiol. 1995. PMID: 7784507 Free PMC article.
-
The Protein Disulfide Isomerase gene family in bread wheat (T. aestivum L.).BMC Plant Biol. 2010 Jun 3;10:101. doi: 10.1186/1471-2229-10-101. BMC Plant Biol. 2010. PMID: 20525253 Free PMC article.
-
Identification and characterization of GmPDIL7, a soybean ER membrane-bound protein disulfide isomerase family protein.FEBS J. 2017 Feb;284(3):414-428. doi: 10.1111/febs.13984. Epub 2016 Dec 25. FEBS J. 2017. PMID: 27960051
-
Characterization of Genes Encoding Protein Disulfide Isomerase in Wheat Including the Specific Role of PDI in the Formation of Gluten.Protein Pept Lett. 2016;23(10):942-950. doi: 10.2174/0929866523666160816145028. Protein Pept Lett. 2016. PMID: 27538701 Review.
-
Oxidative protein folding in the plant endoplasmic reticulum.Biosci Biotechnol Biochem. 2019 May;83(5):781-793. doi: 10.1080/09168451.2019.1571900. Epub 2019 Feb 2. Biosci Biotechnol Biochem. 2019. PMID: 30712483 Review.
Cited by
-
Cooperative Protein Folding by Two Protein Thiol Disulfide Oxidoreductases and 1 in Soybean.Plant Physiol. 2016 Feb;170(2):774-89. doi: 10.1104/pp.15.01781. Epub 2015 Dec 8. Plant Physiol. 2016. PMID: 26645455 Free PMC article.
-
Identification and Functional Analysis of a Protein Disulfide Isomerase (AtPDI1) in Arabidopsis thaliana.Front Plant Sci. 2018 Jul 19;9:913. doi: 10.3389/fpls.2018.00913. eCollection 2018. Front Plant Sci. 2018. PMID: 30073003 Free PMC article.
-
Identification and expression of CAMTA genes in Populus trichocarpa under biotic and abiotic stress.Sci Rep. 2017 Dec 20;7(1):17910. doi: 10.1038/s41598-017-18219-8. Sci Rep. 2017. PMID: 29263356 Free PMC article.
-
Dynamic proteomic changes in soft wheat seeds during accelerated ageing.PeerJ. 2018 Nov 2;6:e5874. doi: 10.7717/peerj.5874. eCollection 2018. PeerJ. 2018. PMID: 30405971 Free PMC article.
-
Gliadins from wheat grain: an overview, from primary structure to nanostructures of aggregates.Biophys Rev. 2018 Apr;10(2):435-443. doi: 10.1007/s12551-017-0367-2. Epub 2017 Dec 4. Biophys Rev. 2018. PMID: 29204878 Free PMC article. Review.
References
-
- Galili G, Shimoni Y, GioriniSilfen S, Levanony H, Altschuler Y, Shani N. Wheat storage proteins: Assembly, transport and deposition in protein bodies. Plant Physiol Biochem. 1996;34:245–52.
-
- Shewry PR, Tatham AS. Disulphide bonds in wheat gluten proteins. J Cereal Sci. 1997;25:207–27. doi: 10.1006/jcrs.1996.0100. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

