Role of glucokinase in the subcellular localization of glucokinase regulatory protein
- PMID: 25849650
- PMCID: PMC4425023
- DOI: 10.3390/ijms16047377
Role of glucokinase in the subcellular localization of glucokinase regulatory protein
Abstract
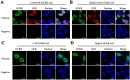
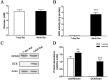
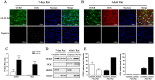
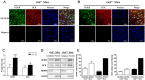
Glucokinase (GCK) is the rate-limiting enzyme of liver glucose metabolism. Through protein-protein interactions, glucokinase regulatory protein (GCKR) post-transcriptionally regulates GCK function in the liver, and causes its nuclear localization. However the role of GCK in regulating GCKR localization is unknown. In the present study, using in vitro and in vivo models, we examined the levels of GCK and GCKR, and their subcellular localization. We found that total cellular levels of GCKR did not vary in the in vivo models, but its subcellular localization did. In animals with normal levels of GCK, GCKR is mainly localized to the nuclei of hepatocytes. In seven-day old rats and liver-specific Gck gene knockout mice (animals that lack or have reduced levels of GCK protein), GCKR was found primarily in the cytoplasm. The interaction of GCK with GCKR was further examined using in vitro models where we varied the levels of GCK and GCKR. Varying the level of GCK protein had no effect on total cellular GCKR protein levels. Taken together, our results indicate that GCK is important for the localization of GCKR to the nucleus and raises the possibility that GCKR may have functions in addition to those regulating GCK activity in the cytoplasm.
Figures
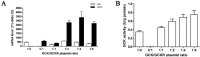
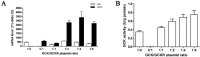



References
-
- Van Schaftingen E. A protein from rat liver confers to glucokinase the property of being antagonistically regulated by fructose 6-phosphate and fructose 1-phosphate. Eur. J. Biochem. 1989;179:179–184. - PubMed
-
- Farrelly D., Brown K.S., Tieman A., Ren J., Lira S.A., Hagan D., Gregg R., Mookhtiar K.A., Hariharan N. Mice mutant for glucokinase regulatory protein exhibit decreased liver glucokinase: A sequestration mechanism in metabolic regulation. Proc. Natl. Acad. Sci. USA. 1999;96:14511–14516. doi: 10.1073/pnas.96.25.14511. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

