Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: a prospective window of opportunity neoadjuvant study
- PMID: 25849721
- PMCID: PMC4381495
- DOI: 10.1186/s13058-015-0540-0
Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: a prospective window of opportunity neoadjuvant study
Abstract
Introduction: The antidiabetic drug metformin exhibits potential anticancer properties that are believed to involve both direct (insulin-independent) and indirect (insulin-dependent) actions. Direct effects are linked to activation of AMP-activated protein kinase (AMPK) and an inhibition of mammalian target of rapamycin mTOR signaling, and indirect effects are mediated by reductions in circulating insulin, leading to reduced insulin receptor (IR)-mediated signaling. However, the in vivo impact of metformin on cancer cell signaling and the factors governing sensitivity in patients remain unknown.
Methods: We conducted a neoadjuvant, single-arm, "window of opportunity" trial to examine the clinical and biological effects of metformin on patients with breast cancer. Women with untreated breast cancer who did not have diabetes were given 500 mg of metformin three times daily for ≥2 weeks after diagnostic biopsy until surgery. Fasting blood and tumor samples were collected at diagnosis and surgery. Blood glucose and insulin were assayed to assess the physiologic effects of metformin, and immunohistochemical analysis of tumors was used to characterize cellular markers before and after treatment.
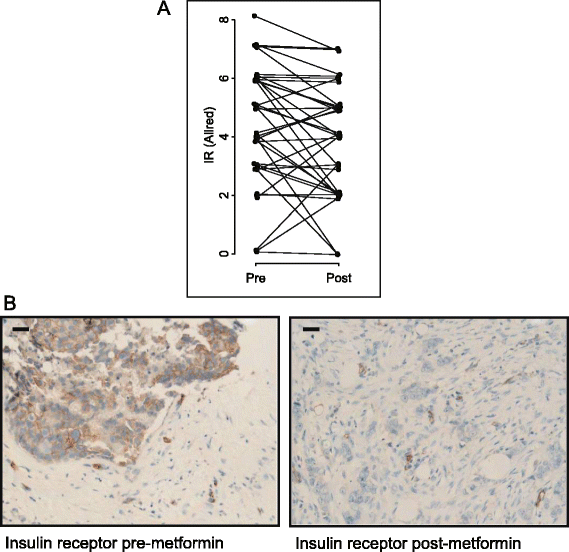
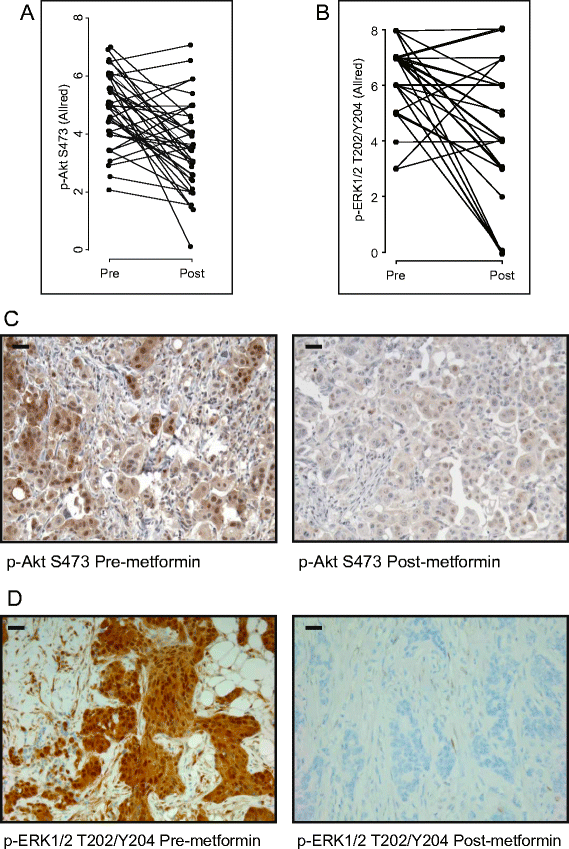
Results: Levels of IR expression decreased significantly in tumors (P = 0.04), as did the phosphorylation status of protein kinase B (PKB)/Akt (S473), extracellular signal-regulated kinase 1/2 (ERK1/2, T202/Y204), AMPK (T172) and acetyl coenzyme A carboxylase (S79) (P = 0.0001, P < 0.0001, P < 0.005 and P = 0.02, respectively). All tumors expressed organic cation transporter 1, with 90% (35 of 39) exhibiting an Allred score of 5 or higher.
Conclusions: Reduced PKB/Akt and ERK1/2 phosphorylation, coupled with decreased insulin and IR levels, suggest insulin-dependent effects are important in the clinical setting. These results are consistent with beneficial anticancer effects of metformin and highlight key factors involved in sensitivity, which could be used to identify patients with breast cancer who may be responsive to metformin-based therapies.
Trial registration: ClinicalTrials.gov identifier: NCT00897884. Registered 8 May 2009.
Figures



Comment in
-
Metformin in breast cancer - an evolving mystery.Breast Cancer Res. 2015 Jun 26;17(1):88. doi: 10.1186/s13058-015-0598-8. Breast Cancer Res. 2015. PMID: 26111812 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

