Synthesis, characterization, crystal structure and antimicrobial activity of copper(II) complexes with the Schiff base derived from 2-hydroxy-4-methoxybenzaldehyde
- PMID: 25849802
- PMCID: PMC6272500
- DOI: 10.3390/molecules20045771
Synthesis, characterization, crystal structure and antimicrobial activity of copper(II) complexes with the Schiff base derived from 2-hydroxy-4-methoxybenzaldehyde
Abstract
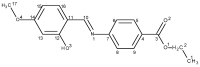
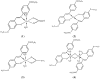
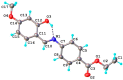
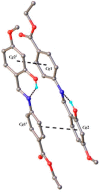
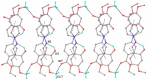
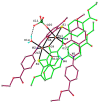
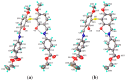
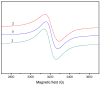
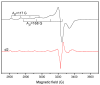
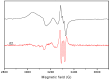
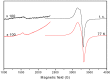
A novel Schiff base, ethyl 4-[(E)-(2-hydroxy-4-methoxyphenyl)methylene-amino]benzoate (HL), was prepared and structurally characterized on the basis of elemental analyses, (1)H NMR, (13)C NMR, UV-Vis and IR spectral data. Six new copper(II) complexes, [Cu(L)(NO3)(H2O)2] (1), [Cu(L)2] (2), [Cu(L)(OAc)] (3), [Cu2 (L)2Cl2(H2O)4] (4), [Cu(L)(ClO4)(H2O)] (5) and [Cu2(L2S)(ClO4)(H2O)]ClO4·H2O (6) have been synthesized. The characterization of the newly formed compounds was done by IR, UV-Vis, EPR, FAB mass spectroscopy, elemental and thermal analysis, magnetic susceptibility measurements and molar electric conductivity. The crystal structures of Schiff base and the complex [Cu2(L2S)(ClO4)(H2O)]ClO4·H2O (6) have been determined by single crystal X-ray diffraction studies. Both copper atoms display a distorted octahedral coordination type [O4NS]. This coordination is ensured by three phenol oxygen, two of which being related to the µ-oxo-bridge, the nitrogen atoms of the azomethine group and the sulfur atoms that come from the polydentate ligand. The in vitro antimicrobial activity against Escherichia coli ATCC 25922, Salmonella enteritidis, Staphylococcus aureus ATCC 25923, Enterococcus and Candida albicans strains was studied and compared with that of free ligand. The complexes 1, 2, 5 showed a better antimicrobial activity than the Schiff base against the tested microorganisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang P.H., Keck J.G., Lien E.J., Lai M.M.C. Design, synthesis, testing and quantitative structure-activity relationship analysis of substituted salicylaldehyde Schiff bases of 1-amino-3hydroxyguanidine tosylate as new antiviral agents against coronavirus. J. Med. Chem. 1990;33:608–614. doi: 10.1021/jm00164a023. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

