Oxidation by neutrophils-derived HOCl increases immunogenicity of proteins by converting them into ligands of several endocytic receptors involved in antigen uptake by dendritic cells and macrophages
- PMID: 25849867
- PMCID: PMC4388828
- DOI: 10.1371/journal.pone.0123293
Oxidation by neutrophils-derived HOCl increases immunogenicity of proteins by converting them into ligands of several endocytic receptors involved in antigen uptake by dendritic cells and macrophages
Erratum in
-
Correction: Oxidation by Neutrophils-Derived HOCl Increases Immunogenicity of Proteins by Converting Them into Ligands of Several Endocytic Receptors Involved in Antigen Uptake by Dendritic Cells and Macrophages.PLoS One. 2015 May 14;10(5):e0128612. doi: 10.1371/journal.pone.0128612. eCollection 2015. PLoS One. 2015. PMID: 25974176 Free PMC article. No abstract available.
Abstract
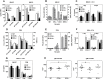
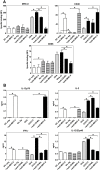
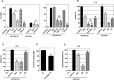
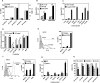
The initiation of adaptive immune responses to protein antigens has to be preceded by their uptake by antigen presenting cells and intracellular proteolytic processing. Paradoxically, endocytic receptors involved in antigen uptake do not bind the majority of proteins, which may be the main reason why purified proteins stimulate at most weak immune responses. A shared feature of different types of adjuvants, capable of boosting immunogenicity of protein vaccines, is their ability to induce acute inflammation, characterized by early influx of activated neutrophils. Neutrophils are also rapidly recruited to sites of tissue injury or infection. These cells are the source of potent oxidants, including hypochlorous acid (HOCl), causing oxidation of proteins present in inflammatory foci. We demonstrate that oxidation of proteins by endogenous, neutrophils-derived HOCl increases their immunogenicity. Upon oxidation, different, randomly chosen simple proteins (yeast alcohol dehydrogenase, human and bovine serum albumin) and glycoproteins (human apo-transferrin, ovalbumin) gain the ability to bind with high affinity to several endocytic receptors on antigen presenting cells, which seems to be the major mechanism of their increased immunogenicity. The mannose receptor (CD206), scavenger receptors A (CD204) and CD36 were responsible for the uptake and presentation of HOCl-modified proteins by murine dendritic cells and macrophages. Other scavenger receptors, SREC-I and LOX-1, as well as RAGE were also able to bind HOCl-modified proteins, but they did not contribute significantly to these ligands uptake by dendritic cells because they were either not expressed or exhibited preference for more heavily oxidised proteins. Our results indicate that oxidation by neutrophils-derived HOCl may be a physiological mechanism of conferring immunogenicity on proteins which in their native forms do not bind to endocytic receptors. This mechanism might enable the immune system to detect infections caused by pathogens not recognized by pattern recognition receptors.
Conflict of interest statement
Figures








Similar articles
-
N-glycosylation converts non-glycoproteins into mannose receptor ligands and reveals antigen-specific T cell responses in vivo.Oncotarget. 2017 Jan 24;8(4):6857-6872. doi: 10.18632/oncotarget.14314. Oncotarget. 2017. PMID: 28036287 Free PMC article.
-
Hypochlorous acid-modified human serum albumin suppresses MHC class II - dependent antigen presentation in pro-inflammatory macrophages.Redox Biol. 2021 Jul;43:101981. doi: 10.1016/j.redox.2021.101981. Epub 2021 Apr 20. Redox Biol. 2021. PMID: 33940547 Free PMC article.
-
Soluble RAGE blocks scavenger receptor CD36-mediated uptake of hypochlorite-modified low-density lipoprotein.FASEB J. 2007 Oct;21(12):3075-82. doi: 10.1096/fj.07-8316com. Epub 2007 May 29. FASEB J. 2007. PMID: 17536039 Free PMC article.
-
Pattern recognition receptors: doubling up for the innate immune response.Cell. 2002 Dec 27;111(7):927-30. doi: 10.1016/s0092-8674(02)01201-1. Cell. 2002. PMID: 12507420 Review.
-
Neutrophils as Sentinel Cells of the Immune System: A Role of the MPO-halide-system in Innate and Adaptive Immunity.Curr Med Chem. 2020;27(17):2840-2851. doi: 10.2174/0929867326666190819123300. Curr Med Chem. 2020. PMID: 31424363 Review.
Cited by
-
Current State of Cold Atmospheric Plasma and Cancer-Immunity Cycle: Therapeutic Relevance and Overcoming Clinical Limitations Using Hydrogels.Adv Sci (Weinh). 2023 Mar;10(8):e2205803. doi: 10.1002/advs.202205803. Epub 2023 Jan 20. Adv Sci (Weinh). 2023. PMID: 36670068 Free PMC article. Review.
-
Cold atmospheric plasma is a viable solution for treating orthopedic infection: a review.Biol Chem. 2018 Dec 19;400(1):77-86. doi: 10.1515/hsz-2018-0235. Biol Chem. 2018. PMID: 30138104 Free PMC article. Review.
-
Phagocytosis of live versus killed or fluorescently labeled bacteria by macrophages differ in both magnitude and receptor specificity.Immunol Cell Biol. 2017 May;95(5):424-435. doi: 10.1038/icb.2016.112. Epub 2016 Nov 9. Immunol Cell Biol. 2017. PMID: 27826145
-
Gas Plasma Technology Augments Ovalbumin Immunogenicity and OT-II T Cell Activation Conferring Tumor Protection in Mice.Adv Sci (Weinh). 2021 Mar 8;8(10):2003395. doi: 10.1002/advs.202003395. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026437 Free PMC article.
-
Effects of In Vitro Combination of Nitric Oxide Donors and Hypochlorite on Acanthamoeba castellanii Viability.Transl Vis Sci Technol. 2023 Sep 1;12(9):23. doi: 10.1167/tvst.12.9.23. Transl Vis Sci Technol. 2023. PMID: 37768280 Free PMC article.
References
-
- Tan MC, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJ, Verwoerd D, et al. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol. 1997;27: 2426–2435. - PubMed
-
- Tagliani E, Guermonprez P, Sepúlveda J, López-Bravo M, Ardavín C, Amigorena S, et al. Selection of an antibody library identifies a pathway to induce immunity by targeting CD36 on steady-state CD8α+ dendritic cells. J Immunol. 2008;180: 3201–3209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

