Fatty acyl donor selectivity in membrane bound O-acyltransferases and communal cell fate decision-making
- PMID: 25849923
- PMCID: PMC4404301
- DOI: 10.1042/BST20140282
Fatty acyl donor selectivity in membrane bound O-acyltransferases and communal cell fate decision-making
Abstract
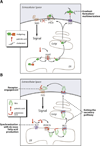
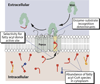
The post-translational modification of proteins with lipid moieties confers spatial and temporal control of protein function by restricting their subcellular distribution or movement in the extracellular milieu. Yet, little is known about the significance of lipid selectivity to the activity of proteins targeted for such modifications. Membrane bound O-acyl transferases (MBOATs) are a superfamily of multipass enzymes that transfer fatty acids on to lipid or protein substrates. Three MBOATs constitute a subfamily with secreted signalling molecules for substrates, the Wnt, Hedgehog (Hh) and Ghrelin proteins. Given their important roles in adult tissue homoeostasis, all three molecules and their respective associated acyltransferases provide a framework for interrogating the role of extracellular acylation events in cell-to-cell communication. Here, we discuss how the preference for a fatty acyl donor in the Wnt acyltransferase porcupine (Porcn) and possibly in other protein lipidation enzymes may provide a means for coupling metabolic health at the single cell level to communal cell fate decision-making in complex multicellular organisms.
Figures
Similar articles
-
An in vitro fatty acylation assay reveals a mechanism for Wnt recognition by the acyltransferase Porcupine.J Biol Chem. 2017 Aug 18;292(33):13507-13513. doi: 10.1074/jbc.C117.800136. Epub 2017 Jun 27. J Biol Chem. 2017. PMID: 28655768 Free PMC article.
-
Stereoselective fatty acylation is essential for the release of lipidated WNT proteins from the acyltransferase Porcupine (PORCN).J Biol Chem. 2019 Apr 19;294(16):6273-6282. doi: 10.1074/jbc.RA118.007268. Epub 2019 Feb 8. J Biol Chem. 2019. PMID: 30737280 Free PMC article.
-
Membrane bound O-acyltransferases and their inhibitors.Biochem Soc Trans. 2015 Apr;43(2):246-52. doi: 10.1042/BST20150018. Biochem Soc Trans. 2015. PMID: 25849925 Review.
-
In vitro reconstitution of Wnt acylation reveals structural determinants of substrate recognition by the acyltransferase human Porcupine.J Biol Chem. 2019 Jan 4;294(1):231-245. doi: 10.1074/jbc.RA118.005746. Epub 2018 Nov 12. J Biol Chem. 2019. PMID: 30420431 Free PMC article.
-
Fatty acylation of proteins: The long and the short of it.Prog Lipid Res. 2016 Jul;63:120-31. doi: 10.1016/j.plipres.2016.05.002. Epub 2016 May 24. Prog Lipid Res. 2016. PMID: 27233110 Free PMC article. Review.
Cited by
-
Targeting Wnt Signaling via Notch in Intestinal Carcinogenesis.Cancers (Basel). 2019 Apr 18;11(4):555. doi: 10.3390/cancers11040555. Cancers (Basel). 2019. PMID: 31003440 Free PMC article. Review.
-
An in vitro fatty acylation assay reveals a mechanism for Wnt recognition by the acyltransferase Porcupine.J Biol Chem. 2017 Aug 18;292(33):13507-13513. doi: 10.1074/jbc.C117.800136. Epub 2017 Jun 27. J Biol Chem. 2017. PMID: 28655768 Free PMC article.
-
Wnt Lipidation and Modifiers in Intestinal Carcinogenesis and Cancer.Cancers (Basel). 2016 Jul 18;8(7):69. doi: 10.3390/cancers8070069. Cancers (Basel). 2016. PMID: 27438855 Free PMC article. Review.
-
Stereoselective fatty acylation is essential for the release of lipidated WNT proteins from the acyltransferase Porcupine (PORCN).J Biol Chem. 2019 Apr 19;294(16):6273-6282. doi: 10.1074/jbc.RA118.007268. Epub 2019 Feb 8. J Biol Chem. 2019. PMID: 30737280 Free PMC article.
-
Monitoring Wnt Protein Acylation Using an In Vitro Cyclo-Addition Reaction.Methods Mol Biol. 2016;1481:11-6. doi: 10.1007/978-1-4939-6393-5_2. Methods Mol Biol. 2016. PMID: 27590147 Free PMC article.
References
-
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer. 2007;7:295–308. - PubMed
-
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat. Rev. 2007;8:74–84. - PubMed
-
- Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem. Sci. 2004;36:245–253. - PubMed
-
- Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu. Rev. Biochem. 2004;73:891–923. - PubMed
-
- Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

