The role of glucocorticoid receptor-dependent activity in the amygdala central nucleus and reversibility of early-life stress programmed behavior
- PMID: 25849981
- PMCID: PMC4462600
- DOI: 10.1038/tp.2015.35
The role of glucocorticoid receptor-dependent activity in the amygdala central nucleus and reversibility of early-life stress programmed behavior
Abstract
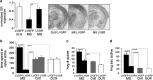
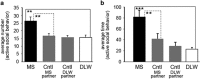
Early-life stress (ELS) leads to sustained changes in gene expression and behavior, increasing the likelihood of developing a psychiatric disorder in adulthood. The neurobiological basis for the later-in-life psychopathology is relatively unknown. The current study used a mouse model of ELS, achieved by daily maternal separations during the first 2 weeks of postnatal life, to test the role of amygdalar glucocorticoid receptor (GR) function in mediating the persistent increase in risk-taking behaviors. ELS produced a decrease in GR mRNA in the brain, with a notable reduction in the amygdala that was associated with sustained alterations in anxiety, fear and sociability-like behaviors. Lentiviral-mediated restoration of the GR mRNA deficit, specifically within the adult central nucleus of the amygdala (CeA), reversed the enduring changes in anxiety and social behavior after ELS. These results provide evidence of lasting changes in CeA GR neural circuitry following ELS and suggest a mechanistic role for GR-regulated processes in the CeA in mediating the lifelong maladaptive behaviors of ELS. We demonstrate that the long-lasting behavioral effects of ELS are reversible later in life and implicate the involvement of CeA GR-dependent activity in the sustained dysregulation of emotion following ELS.
Figures


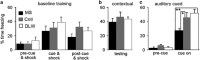
References
-
- Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–1020. - PubMed
-
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Childhood parental loss and adult psychopathology in women. A twin study perspective. Arch Gen Psychiatry. 1992;49:109–116. - PubMed
-
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. - PubMed
-
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

