Identification of receptor-type protein tyrosine phosphatase μ as a new marker for osteocytes
- PMID: 25850409
- PMCID: PMC4468792
- DOI: 10.1007/s00418-015-1319-1
Identification of receptor-type protein tyrosine phosphatase μ as a new marker for osteocytes
Abstract
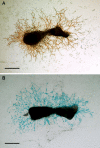
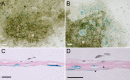
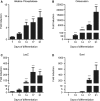
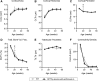
Osteocytes are the predominant cells in bone, where they form a cellular network and display important functions in bone homeostasis, phosphate metabolism and mechanical transduction. Several proteins strongly expressed by osteocytes are involved in these processes, e.g., sclerostin, DMP-1, PHEX, FGF23 and MEPE, while others are upregulated during differentiation of osteoblasts into osteocytes, e.g., osteocalcin and E11. The receptor-type protein tyrosine phosphatase µ (RPTPμ) has been described to be expressed in cells which display a cellular network, e.g., endothelial and neuronal cells, and is implied in mechanotransduction. In a capillary outgrowth assay using metatarsals derived from RPTPμ-knock-out/LacZ knock-in mice, we observed that the capillary structures grown out of the metatarsals were stained blue, as expected. Surprisingly, cells within the metatarsal bone tissue were positive for LacZ activity as well, indicating that RPTPμ is also expressed by osteocytes. Subsequent histochemical analysis showed that within bone, RPTPμ is expressed exclusively in early-stage osteocytes. Analysis of bone marrow cell cultures revealed that osteocytes are present in the nodules and an enzymatic assay enabled the quantification of the amount of osteocytes. No apparent bone phenotype was observed when tibiae of RPTPμ-knock-out/LacZ knock-in mice were analyzed by μCT at several time points during aging, although a significant reduction in cortical bone was observed in RPTPμ-knock-out/LacZ knock-in mice at 20 weeks. Changes in trabecular bone were more subtle. Our data show that RPTPμ is a new marker for osteocytes.
Figures


References
-
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

