Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update
- PMID: 25850553
- PMCID: PMC4520755
- DOI: 10.1038/nrclinonc.2015.61
Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update
Abstract
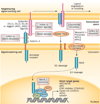
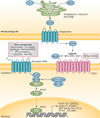
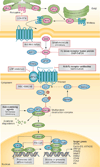
During the past decade, cancer stem cells (CSCs) have been increasingly identified in many malignancies. Although the origin and plasticity of these cells remain controversial, tumour heterogeneity and the presence of small populations of cells with stem-like characteristics is established in most malignancies. CSCs display many features of embryonic or tissue stem cells, and typically demonstrate persistent activation of one or more highly conserved signal transduction pathways involved in development and tissue homeostasis, including the Notch, Hedgehog (HH), and Wnt pathways. CSCs generally have slow growth rates and are resistant to chemotherapy and/or radiotherapy. Thus, new treatment strategies targeting these pathways to control stem-cell replication, survival and differentiation are under development. Herein, we provide an update on the latest advances in the clinical development of such approaches, and discuss strategies for overcoming CSC-associated primary or acquired resistance to cancer treatment. Given the crosstalk between the different embryonic developmental signalling pathways, as well as other pathways, designing clinical trials that target CSCs with rational combinations of agents to inhibit possible compensatory escape mechanisms could be of particular importance. We also share our views on the future directions for targeting CSCs to advance the clinical development of these classes of agents.
Figures
References
-
- Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells—old concepts, new insights. Cell Death Differ. 2008;15:947–958. - PubMed
-
- Espinoza I, Miele L. Deadly crosstalk: notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 2013;341:41–45. - PubMed
-
- Krivtsov AV, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

