Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis
- PMID: 25850656
- PMCID: PMC4598274
- DOI: 10.1038/mi.2015.22
Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis
Erratum in
-
Erratum: Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis.Mucosal Immunol. 2015 May;8(3):699. doi: 10.1038/mi.2015.41. Mucosal Immunol. 2015. PMID: 25943416 No abstract available.
Abstract
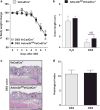
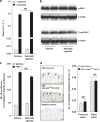
Central to inflammatory bowel disease (IBD) pathogenesis is loss of mucosal barrier function. Emerging evidence implicates extracellular adenosine signaling in attenuating mucosal inflammation. We hypothesized that adenosine-mediated protection from intestinal barrier dysfunction involves tissue-specific signaling through the A2B adenosine receptor (Adora2b) at the intestinal mucosal surface. To address this hypothesis, we combined pharmacologic studies and studies in mice with global or tissue-specific deletion of the Adora2b receptor. Adora2b(-/-) mice experienced a significantly heightened severity of colitis, associated with a more acute onset of disease and loss of intestinal epithelial barrier function. Comparison of mice with Adora2b deletion on vascular endothelial cells (Adora2b(fl/fl)VeCadCre(+)) or intestinal epithelia (Adora2b(fl/fl)VillinCre(+)) revealed a selective role for epithelial Adora2b signaling in attenuating colonic inflammation. In vitro studies with Adora2b knockdown in intestinal epithelial cultures or pharmacologic studies highlighted Adora2b-driven phosphorylation of vasodilator-stimulated phosphoprotein (VASP) as a specific barrier repair response. Similarly, in vivo studies in genetic mouse models or treatment studies with an Adora2b agonist (BAY 60-6583) recapitulate these findings. Taken together, our results suggest that intestinal epithelial Adora2b signaling provides protection during intestinal inflammation via enhancing mucosal barrier responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54 e42. - PubMed
-
- Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. - PubMed
-
- Impellizzeri D, et al. CGS 21680, an agonist of the adenosine (A2A) receptor, decreases acute lung inflammation. Eur J Pharmacol. 2011;668:305–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL114457/HL/NHLBI NIH HHS/United States
- R01-HL119837/HL/NHLBI NIH HHS/United States
- K01 DK099485/DK/NIDDK NIH HHS/United States
- R01 HL098294/HL/NHLBI NIH HHS/United States
- R01 HL080396/HL/NHLBI NIH HHS/United States
- K24 DK100303/DK/NIDDK NIH HHS/United States
- R01-HL098294/HL/NHLBI NIH HHS/United States
- R01 DK103712/DK/NIDDK NIH HHS/United States
- P01-HL114457/HL/NHLBI NIH HHS/United States
- R01-HL092188/HL/NHLBI NIH HHS/United States
- I01 BX002182/BX/BLRD VA/United States
- R01 DK097075/DK/NIDDK NIH HHS/United States
- R37 DK050189/DK/NIDDK NIH HHS/United States
- R01 DK095491/DK/NIDDK NIH HHS/United States
- R01 HL092188/HL/NHLBI NIH HHS/United States
- R01 HL119837/HL/NHLBI NIH HHS/United States
- R01 DK104713/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

