Electroporation Knows No Boundaries: The Use of Electrostimulation for siRNA Delivery in Cells and Tissues
- PMID: 25851034
- PMCID: PMC4543902
- DOI: 10.1177/1087057115579638
Electroporation Knows No Boundaries: The Use of Electrostimulation for siRNA Delivery in Cells and Tissues
Abstract
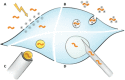
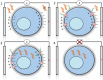
The discovery of RNA interference (RNAi) has enabled several breakthrough discoveries in the area of functional genomics. The RNAi technology has emerged as one of the major tools for drug target identification and has been steadily improved to allow gene manipulation in cell lines, tissues, and whole organisms. One of the major hurdles for the use of RNAi in high-throughput screening has been delivery to cells and tissues. Some cell types are refractory to high-efficiency transfection with standard methods such as lipofection or calcium phosphate precipitation and require different means. Electroporation is a powerful and versatile method for delivery of RNA, DNA, peptides, and small molecules into cell lines and primary cells, as well as whole tissues and organisms. Of particular interest is the use of electroporation for delivery of small interfering RNA oligonucleotides and clustered regularly interspaced short palindromic repeats/Cas9 plasmid vectors in high-throughput screening and for therapeutic applications. Here, we will review the use of electroporation in high-throughput screening in cell lines and tissues.
Keywords: RNA interference; cell transfection; electroporation; high-throughput screening.
© 2015 Society for Laboratory Automation and Screening.
Conflict of interest statement
Figures
Similar articles
-
High efficiency, site-specific transfection of adherent cells with siRNA using microelectrode arrays (MEA).J Vis Exp. 2012 Sep 13;(67):e4415. doi: 10.3791/4415. J Vis Exp. 2012. PMID: 23007885 Free PMC article.
-
High-throughput RNAi screening in vitro: from cell lines to primary cells.RNA. 2005 Jun;11(6):985-93. doi: 10.1261/rna.7288405. RNA. 2005. PMID: 15923380 Free PMC article.
-
Efficient, high-throughput transfection of human embryonic stem cells.Stem Cell Res Ther. 2010 Jul 26;1(3):23. doi: 10.1186/scrt23. Stem Cell Res Ther. 2010. PMID: 20659329 Free PMC article.
-
Targeted electro-delivery of oligonucleotides for RNA interference: siRNA and antimiR.Adv Drug Deliv Rev. 2015 Jan;81:161-8. doi: 10.1016/j.addr.2014.05.002. Epub 2014 May 10. Adv Drug Deliv Rev. 2015. PMID: 24819217 Review.
-
Molecular therapeutics of cancer by means of electroporation-based transfer of siRNAs and EBV-based expression vectors.Front Biosci (Elite Ed). 2009 Jun 1;1(1):316-31. doi: 10.2741/E31. Front Biosci (Elite Ed). 2009. PMID: 19482649 Review.
Cited by
-
Nanocarrier-based systems for targeted and site specific therapeutic delivery.Adv Drug Deliv Rev. 2019 Apr;144:57-77. doi: 10.1016/j.addr.2019.07.010. Epub 2019 Aug 7. Adv Drug Deliv Rev. 2019. PMID: 31400350 Free PMC article. Review.
-
A new age in functional genomics using CRISPR/Cas9 in arrayed library screening.Front Genet. 2015 Sep 24;6:300. doi: 10.3389/fgene.2015.00300. eCollection 2015. Front Genet. 2015. PMID: 26442115 Free PMC article. Review.
-
An Overview of Methods and Tools for Transfection of Eukaryotic Cells in vitro.Front Bioeng Biotechnol. 2021 Jul 20;9:701031. doi: 10.3389/fbioe.2021.701031. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34354988 Free PMC article. Review.
-
Silencing TUBB3 Expression Destroys the Tegument and Flame Cells of Echinococcus multilocularis Protoscoleces.Animals (Basel). 2022 Sep 19;12(18):2471. doi: 10.3390/ani12182471. Animals (Basel). 2022. PMID: 36139331 Free PMC article.
-
Strategies for Delivery of siRNAs to Ovarian Cancer Cells.Pharmaceutics. 2019 Oct 22;11(10):547. doi: 10.3390/pharmaceutics11100547. Pharmaceutics. 2019. PMID: 31652539 Free PMC article. Review.
References
-
- Ketteler R., Glaser S., Sandra O., et al. , Enhanced Transgene Expression in Primitive Hematopoietic Progenitor Cells and Embryonic Stem Cells Efficiently Transduced by Optimized Retroviral Hybrid Vectors. Gene Ther. 2002, 9, 477–487. - PubMed
-
- Schiefsky M. J. Hippocrates on Ancient Medicine. In Studies in Ancient Medicine; Brill, Leiden: Boston, 2005. - PubMed
-
- Kilcher S., Schmidt F. I., Schneider C., et al. siRNA Screen of Early Poxvirus Genes Identifies the AAA+ ATPase D5 as the Virus Genome-Uncoating Factor. Cell Host Microbe 2014, 15, 103–112. - PubMed
-
- Collinet C., Stoter M., Bradshaw C. R., et al. Systems Survey of Endocytosis by Multiparametric Image Analysis. Nature 2010, 464, 243–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

