Overexpression of Nrf2 attenuates Carmustine-induced cytotoxicity in U87MG human glioma cells
- PMID: 25851054
- PMCID: PMC4365816
- DOI: 10.1186/s12885-015-1134-z
Overexpression of Nrf2 attenuates Carmustine-induced cytotoxicity in U87MG human glioma cells
Abstract
Background: Malignant glioma is one of the most devastating tumors in adults with poor patient prognosis. Notably, glioma often exhibits resistance to conventional chemotherapeutic approaches, complicating patient treatments. However, the molecular mediators involved in tumor chemoresistance remain poorly defined, creating a barrier to the successful management of glioma. In the present study, we hypothesized that the antioxidant transcription factor, Nrf2 (nuclear factor erythroid-derived 2 like 2), attenuates glioma cytotoxicity to Carmustine (BCNU), a widely used chemotherapeutic agent known to modulate cellular oxidative balance.
Methods: To test the hypothesis, we employed human malignant glioma cell line, U87MG and overexpression of Nrf2 in glioma cells was achieved using both pharmacological and genetic approaches.
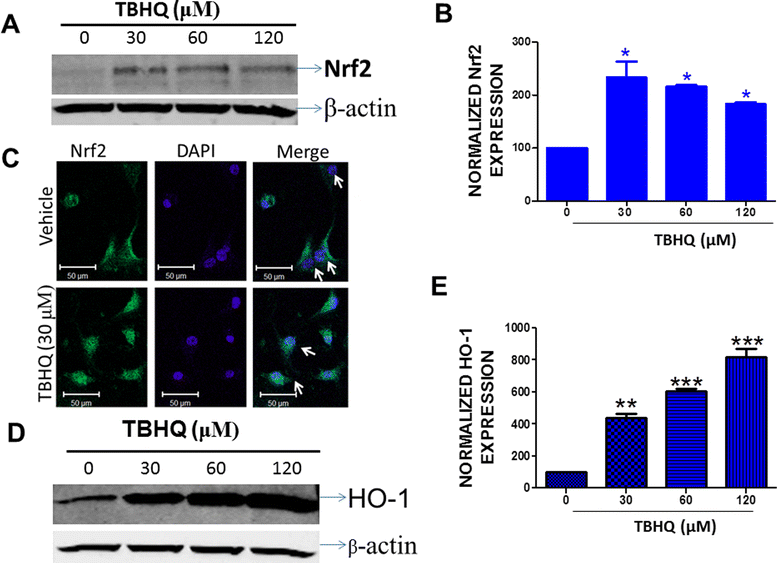
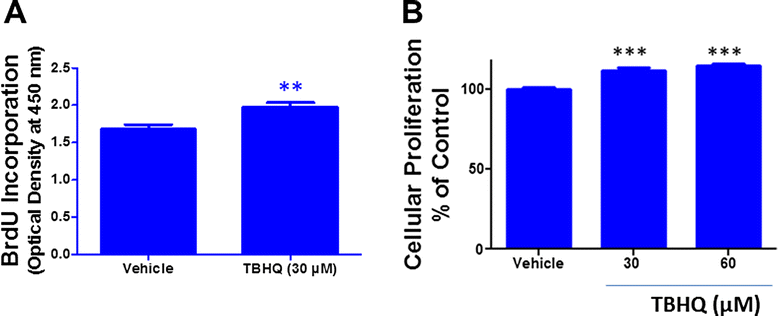
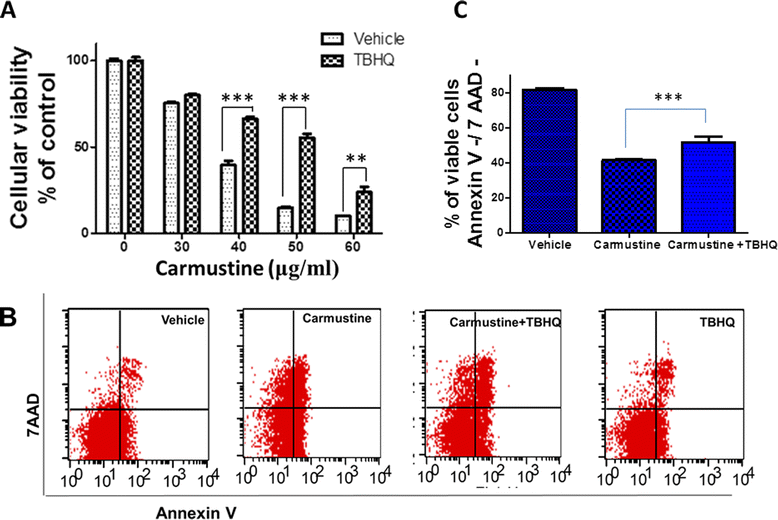
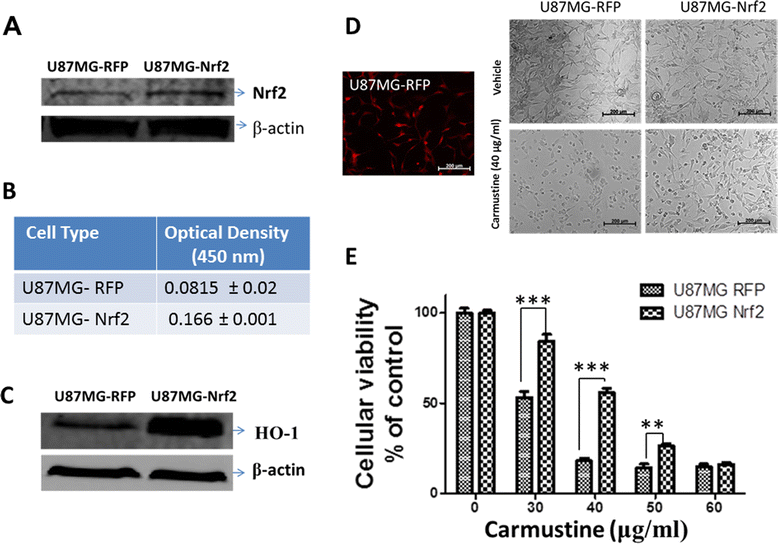
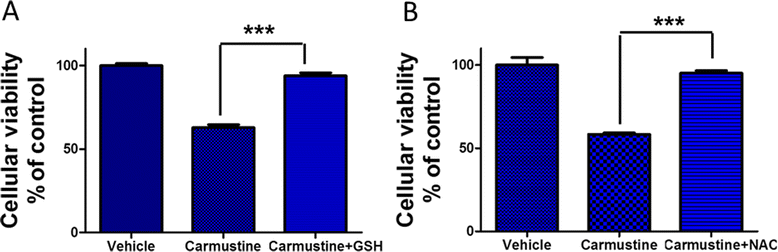
Results: Notably, induction of Nrf2 was associated with increased expression of heme oxygenase-1 (HO-1), a stress inducible enzyme involved in anti-oxidant defense. In addition, over expression of Nrf2 in U87MG cells significantly attenuated the cytotoxicity of Carmustine as evidenced by both cellular viability assay and flow cytometry analysis. Consistent with this, antioxidants such as glutathione and N-acetyl cysteine significantly reduced Carmustine mediated glioma cytotoxicity.
Conclusions: Taken together, these data strongly implicate an unexplored role of Nrf2 in glioma resistance to Carmustine and raise the possible use of Nrf2 inhibitors as adjunct to Carmustine for the treatment of malignant glioma.
Figures
Similar articles
-
Redox proteomics reveal stress responsive proteins linking peroxiredoxin-1 status in glioma to chemosensitivity and oxidative stress.Biochim Biophys Acta. 2015 Jun;1854(6):624-31. doi: 10.1016/j.bbapap.2014.11.011. Epub 2014 Dec 4. Biochim Biophys Acta. 2015. PMID: 25484280
-
p38 MAPK-dependent Nrf2 induction enhances the resistance of glioma cells against TMZ.Med Oncol. 2015 Mar;32(3):69. doi: 10.1007/s12032-015-0517-y. Epub 2015 Feb 19. Med Oncol. 2015. PMID: 25691294
-
Tert-butylhydroquinone as a phenolic activator of Nrf2 antagonizes arsenic-induced oxidative cytotoxicity but promotes arsenic methylation and detoxication in human hepatocyte cell line.Biol Trace Elem Res. 2014 Aug;160(2):294-302. doi: 10.1007/s12011-014-0042-4. Epub 2014 Jun 28. Biol Trace Elem Res. 2014. PMID: 24970285
-
Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1.Free Radic Biol Med. 2014 Feb;67:353-65. doi: 10.1016/j.freeradbiomed.2013.10.819. Epub 2013 Nov 5. Free Radic Biol Med. 2014. PMID: 24200599 Review.
-
Roles of Nrf2 in Gastric Cancer: Targeting for Therapeutic Strategies.Molecules. 2021 May 25;26(11):3157. doi: 10.3390/molecules26113157. Molecules. 2021. PMID: 34070502 Free PMC article. Review.
Cited by
-
UVA Irradiation Enhances Brusatol-Mediated Inhibition of Melanoma Growth by Downregulation of the Nrf2-Mediated Antioxidant Response.Oxid Med Cell Longev. 2018 Feb 18;2018:9742154. doi: 10.1155/2018/9742154. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29670684 Free PMC article.
-
Dissecting the mechanism of temozolomide resistance and its association with the regulatory roles of intracellular reactive oxygen species in glioblastoma.J Biomed Sci. 2021 Mar 8;28(1):18. doi: 10.1186/s12929-021-00717-7. J Biomed Sci. 2021. PMID: 33685470 Free PMC article. Review.
-
A novel prognostic related lncRNA signature associated with amino acid metabolism in glioma.Front Immunol. 2023 Apr 11;14:1014378. doi: 10.3389/fimmu.2023.1014378. eCollection 2023. Front Immunol. 2023. PMID: 37114036 Free PMC article.
-
Post-Injury Administration of Tert-butylhydroquinone Attenuates Acute Neurological Injury After Intracerebral Hemorrhage in Mice.J Mol Neurosci. 2016 Apr;58(4):525-31. doi: 10.1007/s12031-016-0722-y. Epub 2016 Feb 11. J Mol Neurosci. 2016. PMID: 26867538
-
Power frequency magnetic field promotes a more malignant phenotype in neuroblastoma cells via redox-related mechanisms.Sci Rep. 2017 Sep 13;7(1):11470. doi: 10.1038/s41598-017-11869-8. Sci Rep. 2017. PMID: 28904402 Free PMC article.
References
-
- Tamargo RJ, Myseros JS, Epstein JI, Yang MB, Chasin M, Brem H. Interstitial chemotherapy of the 9 L gliosarcoma: controlled release polymers for drug delivery in the brain. Cancer Res. 1993;53(2):329–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

