Extracellular Streptomyces lividans vesicles: composition, biogenesis and antimicrobial activity
- PMID: 25851532
- PMCID: PMC4476819
- DOI: 10.1111/1751-7915.12274
Extracellular Streptomyces lividans vesicles: composition, biogenesis and antimicrobial activity
Abstract
We selected Streptomyces lividans to elucidate firstly the biogenesis and antimicrobial activities of extracellular vesicles that a filamentous and highly differentiated Gram-positive bacterium produces. Vesicle types range in diameter from 110 to 230 nm and 20 to 60 nm, respectively; they assemble to clusters, and contain lipids and phospholipids allowing their in situ imaging by specific fluorescent dyes. The presence of the identified secondary metabolite undecylprodigiosin provokes red fluorescence of a portion of the heterogeneous vesicle populations facilitating in vivo monitoring. Protuberances containing vesicles generate at tips, and alongside of substrate hyphae, and enumerate during late vegetative growth to droplet-like exudates. Owing to in situ imaging in the presence and absence of a green fluorescent vancomycin derivative, we conclude that protuberances comprising vesicles arise at sites with enhanced levels of peptidoglycan subunits [pentapeptide of lipid II (C55)-linked disaccharides], and reduced levels of polymerized and cross-linked peptidoglycan within hyphae. These sites correlate with enhanced levels of anionic phospholipids and lipids. Vesicles provoke pronounced damages of Aspergillus proliferans, Verticillium dahliae and induced clumping and distortion of Escherichia coli. These harmful effects are likely attributable to the action of the identified vesicular compounds including different enzyme types, components of signal transduction cascades and undecylprodigiosin. Based on our pioneering findings, we highlight novel clues with environmental implications and application potential.
© 2015 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Figures


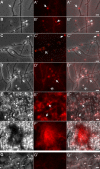
 ), or none (
), or none ( ). Bars: 2.5 μm.G. Aerial hyphae (←) lack intensive red fluorescence (G′ and G″). Bars: 2.5 μm.
). Bars: 2.5 μm.G. Aerial hyphae (←) lack intensive red fluorescence (G′ and G″). Bars: 2.5 μm.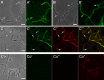
 ).C,C‴. Hyphae were treated with NAO and Nile red, and inspected microscopically under visual light (C), for the presence of NAO derived green fluorescence (C′), for Nile red derived strong red fluorescence (C″), and following merging (C‴) of both types of fluorescence. Pronounced protuberance-structures are marked (
).C,C‴. Hyphae were treated with NAO and Nile red, and inspected microscopically under visual light (C), for the presence of NAO derived green fluorescence (C′), for Nile red derived strong red fluorescence (C″), and following merging (C‴) of both types of fluorescence. Pronounced protuberance-structures are marked ( ).Co–Co*. Untreated control hyphae (Co) lacked green fluorescence (Co′). If analysed under the same gain value that corresponded to C″, the hyphae were not red fluorescent (Co″) showing that the detection of Nile-red dependent staining (C″) was specific. However, if four times higher gain value were applied, some endogenous red fluorescence (Co*) was detectable. Bars: 5 μm.
).Co–Co*. Untreated control hyphae (Co) lacked green fluorescence (Co′). If analysed under the same gain value that corresponded to C″, the hyphae were not red fluorescent (Co″) showing that the detection of Nile-red dependent staining (C″) was specific. However, if four times higher gain value were applied, some endogenous red fluorescence (Co*) was detectable. Bars: 5 μm.
 ) or none (
) or none ( ) are marked. Fluorescence sites (*) that were additionally enlarged are marked. Bars: 2.5 μm.CoA-CoC′. Control (Co) samples corresponding to A, B, and C, which were not treated with Van-FL were analysed under visual light by phase contrast (CoA, CoB and CoC), and inspected for red and green fluorescence light. The resulting pictures were merged. (CoA′, CoB′ and CoC′). Bars: 2.5 μm.
) are marked. Fluorescence sites (*) that were additionally enlarged are marked. Bars: 2.5 μm.CoA-CoC′. Control (Co) samples corresponding to A, B, and C, which were not treated with Van-FL were analysed under visual light by phase contrast (CoA, CoB and CoC), and inspected for red and green fluorescence light. The resulting pictures were merged. (CoA′, CoB′ and CoC′). Bars: 2.5 μm.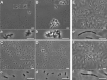

 ) are marked. Regions from the hyphae (B) with high red intracellular fluorescence (*1, or that had developed blown up (*2), or strongly deformed (*3) areas are presented in an enlarged fashion (b1, b2 and b3). Bars 10 μm.D–F. Controls were without the addition of droplets, and inspected as outlined above. Bars 10 μm.
) are marked. Regions from the hyphae (B) with high red intracellular fluorescence (*1, or that had developed blown up (*2), or strongly deformed (*3) areas are presented in an enlarged fashion (b1, b2 and b3). Bars 10 μm.D–F. Controls were without the addition of droplets, and inspected as outlined above. Bars 10 μm.
 ) or no (
) or no ( ) fluorescence. Bars 5 μm.C–E. Pre-grown fungal hyphae were incubated for 20 h with vesicles containing droplets, and inspected as described above. An area (*) of C is presented additionally in an enlarged fashion in c.F–G. Controls correspond to cultures C and E without the addition of droplets, and these were inspected as outlined above. Bars 5 μm.
) fluorescence. Bars 5 μm.C–E. Pre-grown fungal hyphae were incubated for 20 h with vesicles containing droplets, and inspected as described above. An area (*) of C is presented additionally in an enlarged fashion in c.F–G. Controls correspond to cultures C and E without the addition of droplets, and these were inspected as outlined above. Bars 5 μm.References
-
- Aebershold R. Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. - PubMed
-
- Akpe San Roman S, Facey PD, Fernandez-Martinez L, Rodriguez C, Vallin C, Del Sol R. Dyson P. A heterodimer of EsxA and EsxB is involved in sporulation and is secreted by a type VII secretion system in Streptomyces coelicolor. Microbiology. 2010;156:1719–1729. - PubMed
-
- Bardwell JC. Disulfide bond formation, a race between FAD and oxygen. Dev Cell. 2002;3:758–760. - PubMed
-
- Bendtsen JD, Kiemer L, Fausbøll A. Brunak S. Non-classical protein secretion in bacteria. BMC Microbiol. 2005;5:58. and. doi: 10.1186/1471-2180-5-58. - DOI - PMC - PubMed
-
- Berleman JE, Allen S, Danielewicz MA, Remis JP, Gorur A, Cunha J, et al. The lethal cargo of Myxococcus xanthus outer membrane vesicles. Front Microbiol. 2014;5:474. doi: 10.3389/fmicb.2014.00474. eCollection. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

