Mechanisms of fertilization elucidated by gene-manipulated animals
- PMID: 25851662
- PMCID: PMC4492058
- DOI: 10.4103/1008-682X.153299
Mechanisms of fertilization elucidated by gene-manipulated animals
Abstract
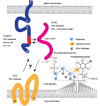
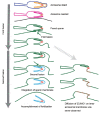
Capacitation and the acrosome reaction are key phenomena in mammalian fertilization. These phenomena were found more than 60 years ago. However, fundamental questions regarding the nature of capacitation and the timing of the acrosome reaction remain unsolved. Factors were postulated over time, but as their roles were not verified by gene-disruption experiments, widely accepted notions concerning the mechanism of fertilization are facing modifications. Today, although in vitro fertilization systems remain our central research tool, the importance of in vivo observations must be revisited. Here, primarily focusing on our own research, I summarize how in vivo observations using gene-manipulated animals have elucidated new concepts in the mechanisms of fertilization.
Figures

References
-
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–96. - PubMed
-
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–8. - PubMed
-
- Dan J. Studies on the acrosome reaction. I. Reaction to egg water and other stimuli. Biol Bull. 1952;103:54–66.
-
- Yanagimachi R, Chang MC. Fertilization of hamster eggs in vitro. Nature. 1963;200:281–2. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

