Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation
- PMID: 25851903
- PMCID: PMC4505563
- DOI: 10.1074/jbc.M115.637439
Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation
Abstract
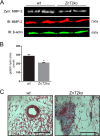
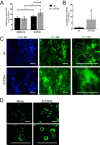
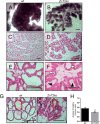
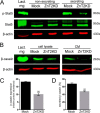
The zinc transporter ZnT2 (SLC30A2) imports zinc into vesicles in secreting mammary epithelial cells (MECs) and is critical for zinc efflux into milk during lactation. Recent studies show that ZnT2 also imports zinc into mitochondria and is expressed in the non-lactating mammary gland and non-secreting MECs, highlighting the importance of ZnT2 in general mammary gland biology. In this study we used nulliparous and lactating ZnT2-null mice and characterized the consequences on mammary gland development, function during lactation, and milk composition. We found that ZnT2 was primarily expressed in MECs and to a limited extent in macrophages in the nulliparous mammary gland and loss of ZnT2 impaired mammary expansion during development. Secondly, we found that lactating ZnT2-null mice had substantial defects in mammary gland architecture and MEC function during secretion, including fewer, condensed and disorganized alveoli, impaired Stat5 activation, and unpolarized MECs. Loss of ZnT2 led to reduced milk volume and milk containing less protein, fat, and lactose compared with wild-type littermates, implicating ZnT2 in the regulation of mammary differentiation and optimal milk production during lactation. Together, these results demonstrate that ZnT2-mediated zinc transport is critical for mammary gland function, suggesting that defects in ZnT2 not only reduce milk zinc concentration but may compromise breast health and increase the risk for lactation insufficiency in lactating women.
Keywords: ZnT2; development; differentiation; lactation; mammary gland; secretion; zinc; zinc transporter.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Zinc transporter 2 interacts with vacuolar ATPase and is required for polarization, vesicle acidification, and secretion in mammary epithelial cells.J Biol Chem. 2017 Dec 29;292(52):21598-21613. doi: 10.1074/jbc.M117.794461. Epub 2017 Nov 7. J Biol Chem. 2017. PMID: 29114036 Free PMC article.
-
Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction.J Mammary Gland Biol Neoplasia. 2015 Dec;20(3-4):159-72. doi: 10.1007/s10911-015-9338-z. Epub 2015 Aug 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26293594
-
ZnT4 (SLC30A4)-null ("lethal milk") mice have defects in mammary gland secretion and hallmarks of precocious involution during lactation.Am J Physiol Regul Integr Comp Physiol. 2016 Jan 1;310(1):R33-40. doi: 10.1152/ajpregu.00315.2014. Epub 2015 Nov 4. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26538236 Free PMC article.
-
The biology of zinc transport in mammary epithelial cells: implications for mammary gland development, lactation, and involution.J Mammary Gland Biol Neoplasia. 2014 Mar;19(1):59-71. doi: 10.1007/s10911-013-9314-4. Epub 2013 Dec 15. J Mammary Gland Biol Neoplasia. 2014. PMID: 24338187 Review.
-
Molecular regulation of lactation: The complex and requisite roles for zinc.Arch Biochem Biophys. 2016 Dec 1;611:86-92. doi: 10.1016/j.abb.2016.04.002. Epub 2016 Apr 6. Arch Biochem Biophys. 2016. PMID: 27059852 Review.
Cited by
-
A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects.Sci Rep. 2018 Feb 23;8(1):3542. doi: 10.1038/s41598-018-21505-8. Sci Rep. 2018. PMID: 29476070 Free PMC article.
-
Zinc transporter 2 interacts with vacuolar ATPase and is required for polarization, vesicle acidification, and secretion in mammary epithelial cells.J Biol Chem. 2017 Dec 29;292(52):21598-21613. doi: 10.1074/jbc.M117.794461. Epub 2017 Nov 7. J Biol Chem. 2017. PMID: 29114036 Free PMC article.
-
Zinc transporters and their functional integration in mammalian cells.J Biol Chem. 2021 Jan-Jun;296:100320. doi: 10.1016/j.jbc.2021.100320. Epub 2021 Jan 22. J Biol Chem. 2021. PMID: 33485965 Free PMC article. Review.
-
Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction.J Mammary Gland Biol Neoplasia. 2015 Dec;20(3-4):159-72. doi: 10.1007/s10911-015-9338-z. Epub 2015 Aug 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26293594
-
Molecular Basis of Transient Neonatal Zinc Deficiency: NOVEL ZnT2 MUTATIONS DISRUPTING ZINC BINDING AND PERMEATION.J Biol Chem. 2016 Jun 24;291(26):13546-59. doi: 10.1074/jbc.M116.732693. Epub 2016 May 2. J Biol Chem. 2016. PMID: 27137936 Free PMC article.
References
-
- Neville M. C. (2009) Introduction: tight junctions and secretory activation in the mammary gland. J. Mammary Gland Biol. Neoplasia 14, 269–270 - PubMed
-
- Brisken C., Rajaram R. D. (2006) Alveolar and lactogenic differentiation. J. Mammary Gland Biol. Neoplasia 11, 239–248 - PubMed
-
- Mutch P. B., Hurley L. S. (1980) Mammary gland function and development: effect of zinc deficiency in rat. Am. J. Physiol. 238, E26–E31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

