Ultrasound molecular imaging: Moving toward clinical translation
- PMID: 25851932
- PMCID: PMC4545409
- DOI: 10.1016/j.ejrad.2015.03.016
Ultrasound molecular imaging: Moving toward clinical translation
Abstract
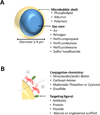
Ultrasound is a widely available, cost-effective, real-time, non-invasive and safe imaging modality widely used in the clinic for anatomical and functional imaging. With the introduction of novel molecularly-targeted ultrasound contrast agents, another dimension of ultrasound has become a reality: diagnosing and monitoring pathological processes at the molecular level. Most commonly used ultrasound molecular imaging contrast agents are micron sized, gas-containing microbubbles functionalized to recognize and attach to molecules expressed on inflamed or angiogenic vascular endothelial cells. There are several potential clinical applications currently being explored including earlier detection, molecular profiling, and monitoring of cancer, as well as visualization of ischemic memory in transient myocardial ischemia, monitoring of disease activity in inflammatory bowel disease, and assessment of arteriosclerosis. Recently, a first clinical grade ultrasound contrast agent (BR55), targeted at a molecule expressed in neoangiogenesis (vascular endothelial growth factor receptor type 2; VEGFR2) has been introduced and safety and feasibility of VEGFR2-targeted ultrasound imaging is being explored in first inhuman clinical trials in various cancer types. This review describes the design of ultrasound molecular imaging contrast agents, imaging techniques, and potential future clinical applications of ultrasound molecular imaging.
Keywords: Cancer; Clinical translation; Inflammation; Microbubbles; Molecular imaging; Ultrasound.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures

Similar articles
-
BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis.Invest Radiol. 2010 Feb;45(2):89-95. doi: 10.1097/RLI.0b013e3181c5927c. Invest Radiol. 2010. PMID: 20027118
-
Dynamic contrast enhanced ultrasound for therapy monitoring.Eur J Radiol. 2015 Sep;84(9):1650-7. doi: 10.1016/j.ejrad.2015.05.013. Epub 2015 Jun 28. Eur J Radiol. 2015. PMID: 26231046 Review.
-
Quantitative ultrasound molecular imaging by modeling the binding kinetics of targeted contrast agent.Phys Med Biol. 2017 Mar 21;62(6):2449-2464. doi: 10.1088/1361-6560/aa5e9a. Epub 2017 Feb 27. Phys Med Biol. 2017. PMID: 28240217
-
Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55.Invest Radiol. 2010 Oct;45(10):573-8. doi: 10.1097/RLI.0b013e3181ee8b83. Invest Radiol. 2010. PMID: 20808233
-
Molecular Ultrasound Imaging.Recent Results Cancer Res. 2020;216:509-531. doi: 10.1007/978-3-030-42618-7_15. Recent Results Cancer Res. 2020. PMID: 32594397 Review.
Cited by
-
Ultrasound Molecular Imaging of the Breast Cancer Neovasculature using Engineered Fibronectin Scaffold Ligands: A Novel Class of Targeted Contrast Ultrasound Agent.Theranostics. 2016 Jun 28;6(11):1740-52. doi: 10.7150/thno.15169. eCollection 2016. Theranostics. 2016. PMID: 27570547 Free PMC article.
-
Ultrasound Molecular Imaging of Bladder Cancer via Extradomain B Fibronectin-Targeted Biosynthetic GVs.Int J Nanomedicine. 2023 Aug 29;18:4871-4884. doi: 10.2147/IJN.S412422. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37662687 Free PMC article.
-
Biogenic Imaging Contrast Agents.Adv Sci (Weinh). 2023 Sep;10(25):e2207090. doi: 10.1002/advs.202207090. Epub 2023 Jul 3. Adv Sci (Weinh). 2023. PMID: 37401173 Free PMC article. Review.
-
Recombinantly Expressed Gas Vesicles as Nanoscale Contrast Agents for Ultrasound and Hyperpolarized MRI.AIChE J. 2018 Aug;64(8):2927-2933. doi: 10.1002/aic.16138. Epub 2018 Feb 23. AIChE J. 2018. PMID: 30555168 Free PMC article.
-
Nondestructive Detection of Targeted Microbubbles Using Dual-Mode Data and Deep Learning for Real-Time Ultrasound Molecular Imaging.IEEE Trans Med Imaging. 2020 Oct;39(10):3079-3088. doi: 10.1109/TMI.2020.2986762. Epub 2020 Apr 9. IEEE Trans Med Imaging. 2020. PMID: 32286963 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

