Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease
- PMID: 25852057
- PMCID: PMC4481596
- DOI: 10.1182/blood-2014-08-595470
Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease
Abstract
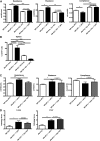
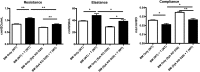
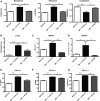
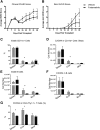
Novel therapies for chronic graft-versus-host disease (cGVHD) are needed. Aberrant B-cell activation has been demonstrated in mice and humans with cGVHD. Having previously found that human cGVHD B cells are activated and primed for survival, we sought to further evaluate the role of the spleen tyrosine kinase (Syk) in cGVHD in multiple murine models and human peripheral blood cells. In a murine model of multiorgan system, nonsclerodermatous disease with bronchiolitis obliterans where cGVHD is dependent on antibody and germinal center (GC) B cells, we found that activation of Syk was necessary in donor B cells, but not T cells, for disease progression. Bone marrow-specific Syk deletion in vivo was effective in treating established cGVHD, as was a small-molecule inhibitor of Syk, fostamatinib, which normalized GC formation and decreased activated CD80/86(+) dendritic cells. In multiple distinct models of sclerodermatous cGVHD, clinical and pathological disease manifestations were not eliminated when mice were therapeutically treated with fostamatinib, though both clinical and immunologic effects could be observed in one of these scleroderma models. We further demonstrated that Syk inhibition was effective at inducing apoptosis of human cGVHD B cells. Together, these data demonstrate a therapeutic potential of targeting B-cell Syk signaling in cGVHD.
© 2015 by The American Society of Hematology.
Figures



Comment in
-
Syk and tired of current chronic GVHD therapies.Blood. 2015 Jun 25;125(26):3974-5. doi: 10.1182/blood-2015-05-640672. Blood. 2015. PMID: 26113534 Free PMC article.
References
-
- Schultz KR, Miklos DB, Fowler D, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12(2):126-137. - PubMed
-
- Shlomchik WD, Lee SJ, Couriel D, Pavletic SZ. Transplantation's greatest challenges: advances in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(1 suppl 1):2-10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

