Rate-of-Change Dependence of the Performance of Two CGM Systems During Induced Glucose Swings
- PMID: 25852074
- PMCID: PMC4525645
- DOI: 10.1177/1932296815578716
Rate-of-Change Dependence of the Performance of Two CGM Systems During Induced Glucose Swings
Abstract
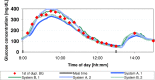
Introduction: The accuracy of continuous glucose monitoring (CGM) systems is often assessed with respect to blood glucose (BG) readings. CGM readings are affected by a physiological and a technical time delay when compared to BG readings. In this analysis, the dependence of CGM performance parameters on the BG rate of change was investigated for 2 CGM systems.
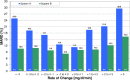
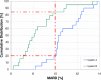
Methods: Data from a previously published study were retrospectively analyzed. An established CGM system (Dexcom G4, Dexcom, San Diego, CA; system A) and a prototype system (Roche Diagnostics GmbH, Mannheim, Germany; system B) with 2 sensors each were worn by 10 subjects in parallel. Glucose swings were induced to achieve rapidly changing BG concentrations. Mean absolute relative differences (MARD) were calculated in different BG rate-of-change categories. In addition, sensor-to-sensor precision was assessed.
Results: At BG rates of change of -1 mg/dl/min to 0 mg/dl/min and 0 mg/dl/min to +1 mg/dl/min, MARD results were 12.6% and 11.3% for system A and 8.2% and 10.0% for system B. At rapidly changing BG concentrations (<-3 mg/dl/min and ≥+3 mg/dl/min), higher MARD results were found for both systems, but system B was less affected (system A: 24.9% and 29.6%, system B: 10.6% and 16.3%). The impact of rate of change on sensor-to-sensor precision was less pronounced.
Conclusions: Both systems were affected by rapidly changing BG concentrations to some degree, although system B was mostly unaffected by decreasing BG concentrations. It would seem that technological advancements in CGM systems might allow for a more precise tracking of BG concentrations even at rapidly changing BG concentrations.
Keywords: MARD; PARD; accuracy; continuous glucose monitoring; rate of change.
© 2015 Diabetes Technology Society.
Conflict of interest statement
Figures
Similar articles
-
Improved Accuracy of Continuous Glucose Monitoring Systems in Pediatric Patients with Diabetes Mellitus: Results from Two Studies.Diabetes Technol Ther. 2016 Feb;18 Suppl 2(Suppl 2):S223-33. doi: 10.1089/dia.2015.0380. Diabetes Technol Ther. 2016. PMID: 26784126 Free PMC article.
-
Performance evaluation of a continuous glucose monitoring system under conditions similar to daily life.J Diabetes Sci Technol. 2013 Jul 1;7(4):833-41. doi: 10.1177/193229681300700405. J Diabetes Sci Technol. 2013. PMID: 23911164 Free PMC article. Clinical Trial.
-
Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring.Diabetes Technol Ther. 2015 Mar;17(3):177-86. doi: 10.1089/dia.2014.0272. Epub 2014 Dec 1. Diabetes Technol Ther. 2015. PMID: 25436913 Free PMC article.
-
Calibration algorithms for continuous glucose monitoring systems based on interstitial fluid sensing.Biosens Bioelectron. 2024 Sep 15;260:116450. doi: 10.1016/j.bios.2024.116450. Epub 2024 May 28. Biosens Bioelectron. 2024. PMID: 38843770 Review.
-
Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices.J Diabetes Sci Technol. 2019 May;13(3):575-583. doi: 10.1177/1932296818812062. Epub 2018 Nov 19. J Diabetes Sci Technol. 2019. PMID: 30453761 Free PMC article. Review.
Cited by
-
Perceptions of Continuous Glucose Monitoring Systems in the T1D Exchange Diabetes Registry: Satisfaction, Concerns, and Areas for Future Improvement.Clin Diabetes. 2024 Winter;42(1):104-115. doi: 10.2337/cd23-0005. Epub 2023 Aug 23. Clin Diabetes. 2024. PMID: 38230340 Free PMC article.
-
Assessing the Accuracy of Continuous Glucose Monitoring (CGM) Calibrated With Capillary Values Using Capillary or Venous Glucose Levels as a Reference.J Diabetes Sci Technol. 2016 Jun 28;10(4):876-84. doi: 10.1177/1932296815626724. Print 2016 Jul. J Diabetes Sci Technol. 2016. PMID: 26810924 Free PMC article. Clinical Trial.
-
Simple Post-Processing of Continuous Glucose Monitoring Measurements Improves Endpoints in Clinical Trials.J Diabetes Sci Technol. 2020 Nov;14(6):1074-1078. doi: 10.1177/1932296819848721. Epub 2019 May 16. J Diabetes Sci Technol. 2020. PMID: 31096765 Free PMC article.
-
Improved glycaemic variability and basal insulin dose reduction during a running competition in recreationally active adults with type 1 diabetes-A single-centre, prospective, controlled observational study.PLoS One. 2020 Sep 11;15(9):e0239091. doi: 10.1371/journal.pone.0239091. eCollection 2020. PLoS One. 2020. PMID: 32915897 Free PMC article.
-
Pilot study in human healthy volunteers on the use of magnetohydrodynamics in needle-free continuous glucose monitoring.Sci Rep. 2022 Nov 9;12(1):18318. doi: 10.1038/s41598-022-21424-9. Sci Rep. 2022. PMID: 36351930 Free PMC article.
References
-
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. - PubMed
-
- IDF Clinical Guidelines Task Force. Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23(6):579-593. - PubMed
-
- Bailey TS, Ahmann A, Brazg R, et al. Accuracy and acceptability of the 6-day Enlite continuous subcutaneous glucose sensor. Diabetes Technol Ther. 2014;16(5):277-283. - PubMed
-
- Klaff LJ, Brazg R, Hughes K, et al. Accuracy evaluation of Contour Next compared with five blood glucose monitoring systems across a wide range of blood glucose concentrations occurring in a clinical research setting. Diabetes Technol Ther. 2015;17:8-15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

