Rate-of-Change Dependence of the Performance of Two CGM Systems During Induced Glucose Swings
- PMID: 25852074
- PMCID: PMC4525645
- DOI: 10.1177/1932296815578716
Rate-of-Change Dependence of the Performance of Two CGM Systems During Induced Glucose Swings
Abstract
Introduction: The accuracy of continuous glucose monitoring (CGM) systems is often assessed with respect to blood glucose (BG) readings. CGM readings are affected by a physiological and a technical time delay when compared to BG readings. In this analysis, the dependence of CGM performance parameters on the BG rate of change was investigated for 2 CGM systems.
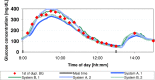
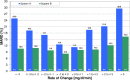
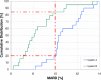
Methods: Data from a previously published study were retrospectively analyzed. An established CGM system (Dexcom G4, Dexcom, San Diego, CA; system A) and a prototype system (Roche Diagnostics GmbH, Mannheim, Germany; system B) with 2 sensors each were worn by 10 subjects in parallel. Glucose swings were induced to achieve rapidly changing BG concentrations. Mean absolute relative differences (MARD) were calculated in different BG rate-of-change categories. In addition, sensor-to-sensor precision was assessed.
Results: At BG rates of change of -1 mg/dl/min to 0 mg/dl/min and 0 mg/dl/min to +1 mg/dl/min, MARD results were 12.6% and 11.3% for system A and 8.2% and 10.0% for system B. At rapidly changing BG concentrations (<-3 mg/dl/min and ≥+3 mg/dl/min), higher MARD results were found for both systems, but system B was less affected (system A: 24.9% and 29.6%, system B: 10.6% and 16.3%). The impact of rate of change on sensor-to-sensor precision was less pronounced.
Conclusions: Both systems were affected by rapidly changing BG concentrations to some degree, although system B was mostly unaffected by decreasing BG concentrations. It would seem that technological advancements in CGM systems might allow for a more precise tracking of BG concentrations even at rapidly changing BG concentrations.
Keywords: MARD; PARD; accuracy; continuous glucose monitoring; rate of change.
© 2015 Diabetes Technology Society.
Conflict of interest statement
Figures
References
-
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. - PubMed
-
- IDF Clinical Guidelines Task Force. Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23(6):579-593. - PubMed
-
- Bailey TS, Ahmann A, Brazg R, et al. Accuracy and acceptability of the 6-day Enlite continuous subcutaneous glucose sensor. Diabetes Technol Ther. 2014;16(5):277-283. - PubMed
-
- Klaff LJ, Brazg R, Hughes K, et al. Accuracy evaluation of Contour Next compared with five blood glucose monitoring systems across a wide range of blood glucose concentrations occurring in a clinical research setting. Diabetes Technol Ther. 2015;17:8-15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

