Exoelectrogenic capacity of host microbiota predicts lymphocyte recruitment to the gut
- PMID: 25852170
- PMCID: PMC4491528
- DOI: 10.1152/physiolgenomics.00010.2015
Exoelectrogenic capacity of host microbiota predicts lymphocyte recruitment to the gut
Abstract
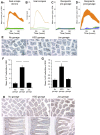
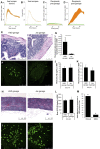
Electrotaxis, directional cell movement in response to an electric potential, has been demonstrated in a wide range of cell types including lymphocytes. Exoelectrogens, microorganisms capable of generating electrical currents, have been identified in microbial fuel cells. However, no studies have investigated exoelectrogenic microbes in fresh feces or the effects of an exoelectrogenic microbiota on the host organism. Here we show that commensal gut microbial populations differ in their capacity for electrical current production by exoelectrogens and that those differences are predictive of increased lymphocyte trafficking to the gut in vivo, despite the lack of increased production of canonical lymphocyte-specific chemokines. Additionally, we demonstrate that the difference in current production between mice purchased from different commercial sources correlates reproducibly with the presence or absence of segmented filamentous bacteria, and while our data do not support a direct role for segmented filamentous bacteria in ex vivo current production, an exoelectrogenic microbiota can be transferred in vivo via mucosa-associated bacteria present in the ileum. Moreover, we detect upregulation of microbial genes associated with extracellular electron transfer in feces of mice colonized with exoelectrogenic microbiota containing segmented filamentous bacteria. While still correlative, these results suggest a novel means by which the gut microbiota modulates the recruitment of cells of the immune system to the gut.
Keywords: electrotaxis; exoelectrogen; microbiota; segmented filamentous bacteria.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Call DF, Logan BE. A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells. Biosens Bioelectron 26: 4526–4531, 2011. - PubMed
-
- Campbell JM, Fahey GC Jr, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr 127: 130–136, 1997. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

