Avian reovirus replication in mononuclear phagocytes in chicken footpad and spleen after footpad inoculation
- PMID: 25852223
- PMCID: PMC4365711
Avian reovirus replication in mononuclear phagocytes in chicken footpad and spleen after footpad inoculation
Abstract
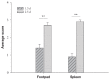
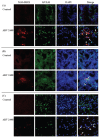
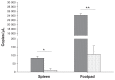
Circulating monocytes and tissue macrophages were suggested to be susceptible to avian reovirus (ARV) infection. To determine if ARV infects and replicates in mononuclear phagocytes (KUL01-positive cells), we infected 3-day-old specific-pathogen-free chickens with ARV strain 2408 by inoculation of the left footpad. The left footpads and spleens were collected for analysis at 1.5 and 2.5 d after inoculation. Replication of ARV in the footpad and spleen was demonstrated by detection of the viral protein σNS using immunohistochemical testing and viral S1 RNA expression by real-time quantitative polymerase chain reaction (qPCR). Furthermore, immunofluorescent double-staining assay of cytocentrifuged cells and cryosections of the footpad and spleen for the viral protein σNS and the surface marker recognized by monoclonal antibody (MAb) KUL01 indicated that KUL01-positive cells costained with MAb H1E1, which recognizes ARV protein σNS. In addition, more ARV S1 RNA was measured by qPCR in the KUL01-positive cell samples prepared from the footpad or spleen 1.5 d after inoculation compared with non-KUL01-positive cell samples. The amounts of ARV S1 RNA in the spleen were significantly lower (P < 0.05) than the amounts in the footpad 1.5 d after inoculation. The results suggest that ARV infects mononuclear phagocytes and then replicates within these cells before migrating to the spleen, where it infects and replicates in KUL01-positive cells.
Il a été suggéré que les monocytes circulants et les macrophages tissulaires étaient sensibles à une infection par le reovirus aviaire (ARV). Afin de déterminer si l’ARV infecte et se réplique dans les phagocytes mononucléaires (cellules KUL01-positives), nous avons infecté des poussins exempts d’agents pathogènes spécifiques âgés de 3 j avec la souche 2408 d’ARV par inoculation dans le coussinet plantaire gauche. Les coussinets plantaires et les rates furent prélevés pour analyse aux jours 1,5 et 2,5 suivant l’inoculation. La réplication d’ARV dans le coussinet plantaire et la rate fut démontrée par détection de la protéine virale σNS par épreuve immunohistochimique et l’expression d’ARN S1 viral par réaction d’amplification en chaîne par la polymérase en temps réel (qPCR). De plus, l’épreuve d’immunofluorescence par double coloration de cellules cytocentrifugées et de coupes congelées du coussinet plantaire et de la rate pour la protéine virale σNS et le marqueur de surface reconnu par l’anticorps monoclonal (AcMo) KUL01 indiquait que les cellules positives pour KUL01se co-coloraient avec l’AcMo H1E1, qui reconnait la protéine σNS de l’ARV. Également, plus d’ARN S1 d’ARV était mesuré par qPCR dans les échantillons de cellules KUL01 positives préparés à partir de coussinets plantaires ou de rates 1,5 j après l’inoculation comparativement à des échantillons de cellules KUL01 négatives. Les quantités d’ARN S1 d’ARV dans la rate étaient significativement plus basses (P < 0,05) que les quantités dans les coussinets plantaires 1,5 j après l’inoculation. Les résultats suggèrent que l’ARV infecte les phagocytes mononucléaires et par la suite se répliquent dans ces cellules avant de migrer à la rate, où il infecte et se réplique dans les cellules KUL01-positives.(Traduit par Docteur Serge Messier).
Figures



Similar articles
-
Antibody responses against avian reovirus nonstructural protein sigmaNS in experimentally virus-infected chickens monitored by a monoclonal antibody capture enzyme-linked immunosorbent assay.Res Vet Sci. 2004 Jun;76(3):219-25. doi: 10.1016/j.rvsc.2003.11.001. Res Vet Sci. 2004. PMID: 15046956
-
Differential expression of U2AF35 in the arthritic joint of avian reovirus-infected chicks.Vet Immunol Immunopathol. 2006 Nov 15;114(1-2):49-60. doi: 10.1016/j.vetimm.2006.07.003. Epub 2006 Aug 17. Vet Immunol Immunopathol. 2006. PMID: 16916547
-
Novel strand-specific qPCR assay elucidates differences in viral replication kinetics between different strains of avian reovirus.Microbiol Spectr. 2025 Jun 3;13(6):e0314024. doi: 10.1128/spectrum.03140-24. Epub 2025 Apr 30. Microbiol Spectr. 2025. PMID: 40304489 Free PMC article.
-
Pathogenicity of avian reovirus variant in the immune organs of broiler chicks.Virus Res. 2025 Mar;353:199538. doi: 10.1016/j.virusres.2025.199538. Epub 2025 Feb 6. Virus Res. 2025. PMID: 39909158 Free PMC article.
-
Avian Reovirus: From Molecular Biology to Pathogenesis and Control.Viruses. 2024 Dec 23;16(12):1966. doi: 10.3390/v16121966. Viruses. 2024. PMID: 39772272 Free PMC article. Review.
Cited by
-
Autophagy induced by avian reovirus enhances viral replication in chickens at the early stage of infection.BMC Vet Res. 2019 May 24;15(1):173. doi: 10.1186/s12917-019-1926-5. BMC Vet Res. 2019. PMID: 31126305 Free PMC article.
-
Virulence of Emerging Arthrotropic Avian Reoviruses Correlates With Their Ability to Activate and Traffic Interferon-γ Producing Cytotoxic CD8+ T Cells Into Gastrocnemius Tendon.Front Microbiol. 2022 Mar 14;13:869164. doi: 10.3389/fmicb.2022.869164. eCollection 2022. Front Microbiol. 2022. PMID: 35369435 Free PMC article.
-
Avian Reoviruses From Wild Birds Exhibit Pathogenicity to Specific Pathogen Free Chickens by Footpad Route.Front Vet Sci. 2022 Feb 24;9:844903. doi: 10.3389/fvets.2022.844903. eCollection 2022. Front Vet Sci. 2022. PMID: 35280152 Free PMC article.
-
Development of a multivalent adjuvanted inactivated vaccine against variant arthrotropic avian reoviruses.Front Vet Sci. 2023 Oct 18;10:1209597. doi: 10.3389/fvets.2023.1209597. eCollection 2023. Front Vet Sci. 2023. PMID: 37920329 Free PMC article.
-
Molecular and pathological investigation of avian reovirus (ARV) in Egypt with the assessment of the genetic variability of field strains compared to vaccine strains.Front Microbiol. 2023 Apr 17;14:1156251. doi: 10.3389/fmicb.2023.1156251. eCollection 2023. Front Microbiol. 2023. PMID: 37138631 Free PMC article.
References
-
- Schnitzer TJ. Protein coding assignment of the S-genes of the avian reovirus-S1133. Virology. 1985;141:167–170. - PubMed
-
- Page RK, Fletcher OJ, Rowland GN, Gaudry D, Villegas P. Malabsorption syndrome in broiler chickens. Avian Dis. 1982;26:618–624. - PubMed
-
- Robertson MD, Wilcox GE. Avian reovirus. Vet Bull. 1986;56:155–174.
-
- Olson NO, Khan MA. The effect of intranasal exposure of chickens to the Fahey-Crawley virus on the development of synovial lesions. Avian Dis. 1972;16:1073–1078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
