Identification of Escherichia coli F4ac-binding proteins in porcine milk fat globule membrane
- PMID: 25852227
- PMCID: PMC4365703
Identification of Escherichia coli F4ac-binding proteins in porcine milk fat globule membrane
Abstract
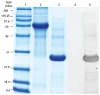
F4ac-positive enterotoxigenic Escherichia coli (ETEC) must attach to the intestinal mucosa to cause diarrhea in piglets. Prevention of bacterial attachment to the intestinal mucosa is the most effective defense against ETEC-induced diarrhea. Porcine milk fat globule membranes (MFGM) were shown to be able to inhibit attachment of ETEC to the intestinal brush border; however, the specific components of porcine MFGM that inhibited attachment of ETEC to enterocytes were not identified. Accordingly, the purpose of this study was to identify F4ac-binding MFGM proteins by overlay Western blot and affinity chromatography. The proteome of porcine MFGM was characterized and the following F4ac-binding proteins were detected by overlay Western blot and affinity chromatography: lactadherin, butyrophilin, adipophilin, acyl-CoA synthetase 3, and fatty acid-binding protein 3. The biological function of these proteins was not investigated but it is possible that their interaction with F4ac fimbria interferes with bacterial attachment and colonization.
Les Escherichia coli entérotoxinogénique (ETEC) positif pour F4ac doivent s’attacher à la muqueuse intestinale pour causer la diarrhée chez les porcelets. L’empêchement de l’attachement bactérien à la muqueuse intestinale est le moyen de défense le plus efficace contre la diarrhée induite par les ETEC. Les membranes de globules de gras de lait porcin (MFGM) ont été montré comme étant capable d’inhiber l’attachement des ETEC à la bordure en brosse intestinale; toutefois, les composantes spécifiques des MFGM porcines qui inhibaient l’attachement des ETEC aux entérocytes ne furent pas identifiées. Ainsi, le but de la présente étude était d’identifier les protéines des MFGM liant F4ac par immunobuvardage par superposition et chromatographie d’affinité. Le protéome des MFGM porcine fut caractérisé et les protéines liant F4as suivantes furent détectées par immunobuvardage par superposition et chromatographie d’affinité : lactadhérine, butyrophiline, adipophiline, acyl-CoA synthétase 3, et la protéine 3 liant les acides gras. La fonction biologique de ces protéines ne fut pas étudiée mais il est possible que leur interaction avec les fimbriae F4ac interfère avec l’attachement bactérien et la colonisation.(Traduit par Docteur Serge Messier).
Figures



References
-
- Hampson DJ, Gyles CL. Postweaning Escherichia coli diarrhoea in pigs. In: Gyles CL, editor. Escherichia coli in Domestic Animals and Humans. Wallingford, England: Cab International; 1994. pp. 171–191.
-
- Shahriar F, Ngeleka M, Gordon JR, Simko E. Identification by mass spectroscopy of F4ac-fimbrial-binding proteins in porcine milk and characterization of lactadherin as an inhibitor of F4ac-positive Escherichia coli attachment to intestinal villi in vitro. Dev Comp Immunol. 2006;30:723–734. - PubMed
-
- Vondruskova H, Slamova R, Trckova M, Zraly Z, Pavlik I. Alternatives to antibiotic growth promoters in prevention of diarrhoea in weaned piglets: A review. Vet Med. 2010;55:199–224.
-
- Atroshi F, Alaviuhkola T, Schildt R, Sandholm M. Fat globule membrane of sow milk as a target for adhesion of K88-positive Escherichia coli. Comp Immunol Microbiol Infect Dis. 1983;6:235–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
