Fucosylation is a common glycosylation type in pancreatic cancer stem cell-like phenotypes
- PMID: 25852272
- PMCID: PMC4385534
- DOI: 10.3748/wjg.v21.i13.3876
Fucosylation is a common glycosylation type in pancreatic cancer stem cell-like phenotypes
Abstract
Aim: To evaluate/isolate cancer stem cells (CSCs) from tissue or cell lines according to various definitions and cell surface markers.
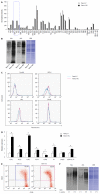
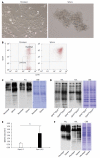
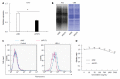
Methods: Lectin microarray analysis was conducted on CSC-like fractions of the human pancreatic cancer cell line Panc1 by establishing anti-cancer drug-resistant cells. Changes in glycan structure of CSC-like cells were also investigated in sphere-forming cells as well as in CSC fractions obtained from overexpression of CD24 and CD44.
Results: Several types of fucosylation were increased under these conditions, and the expression of fucosylation regulatory genes such as fucosyltransferases, GDP-fucose synthetic enzymes, and GDP-fucose transporters were dramatically enhanced in CSC-like cells. These changes were significant in gemcitabine-resistant cells and sphere cells of a human pancreatic cancer cell line, Panc1. However, downregulation of cellular fucosylation by knockdown of the GDP-fucose transporter did not alter gemcitabine resistance, indicating that increased cellular fucosylation is a result of CSC-like transformation.
Conclusion: Fucosylation might be a biomarker of CSC-like cells in pancreatic cancer.
Keywords: Anti-cancer drug resistance; Cancer stem cells; Fucosylation; Glycosylation; Pancreatic cancer; Sphere formation.
Figures



References
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–466. - PubMed
-
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

