Outer brain barriers in rat and human development
- PMID: 25852456
- PMCID: PMC4360706
- DOI: 10.3389/fnins.2015.00075
Outer brain barriers in rat and human development
Abstract
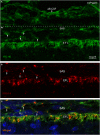
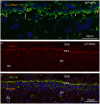
Complex barriers at the brain's surface, particularly in development, are poorly defined. In the adult, arachnoid blood-cerebrospinal fluid (CSF) barrier separates the fenestrated dural vessels from the CSF by means of a cell layer joined by tight junctions. Outer CSF-brain barrier provides diffusion restriction between brain and subarachnoid CSF through an initial radial glial end feet layer covered with a pial surface layer. To further characterize these interfaces we examined embryonic rat brains from E10 to P0 and forebrains from human embryos and fetuses (6-21st weeks post-conception) and adults using immunohistochemistry and confocal microscopy. Antibodies against claudin-11, BLBP, collagen 1, SSEA-4, MAP2, YKL-40, and its receptor IL-13Rα2 and EAAT1 were used to describe morphological characteristics and functional aspects of the outer brain barriers. Claudin-11 was a reliable marker of the arachnoid blood-CSF barrier. Collagen 1 delineated the subarachnoid space and stained pial surface layer. BLBP defined radial glial end feet layer and SSEA-4 and YKL-40 were present in both leptomeningeal cells and end feet layer, which transformed into glial limitans. IL-13Rα2 and EAAT1 were present in the end feet layer illustrating transporter/receptor presence in the outer CSF-brain barrier. MAP2 immunostaining in adult brain outlined the lower border of glia limitans; remnants of end feet were YKL-40 positive in some areas. We propose that outer brain barriers are composed of at least 3 interfaces: blood-CSF barrier across arachnoid barrier cell layer, blood-CSF barrier across pial microvessels, and outer CSF-brain barrier comprising glial end feet layer/pial surface layer.
Keywords: SSEA-4; YKL-40; arachnoid blood-CSF barrier; development; outer CSF-brain barrier; pial microvessel blood-CSF barrier; subarachnoid space.
Figures



Similar articles
-
Ontogenetic development of diffusional restriction to protein at the pial surface of the rat brain: an electron microscopical study.J Neurocytol. 1997 Mar;26(3):133-48. doi: 10.1023/a:1018527928760. J Neurocytol. 1997. PMID: 9192282
-
Barrier mechanisms in the brain, II. Immature brain.Clin Exp Pharmacol Physiol. 1999 Feb;26(2):85-91. doi: 10.1046/j.1440-1681.1999.02987.x. Clin Exp Pharmacol Physiol. 1999. PMID: 10065326 Review.
-
Morphological indications for considerable diffuse reabsorption of cerebrospinal fluid in spinal meninges particularly in the areas of meningeal funnels. An electronmicroscopical study including tracing experiments in rats.Anat Embryol (Berl). 1994 Mar;189(3):243-58. doi: 10.1007/BF00239012. Anat Embryol (Berl). 1994. PMID: 8042766
-
Brain iron homeostasis.Dan Med Bull. 2002 Nov;49(4):279-301. Dan Med Bull. 2002. PMID: 12553165 Review.
-
Response to Commentary on "Structural characterization of SLYM - a 4th meningeal membrane" by Julie Siegenthaler and Christer Betsholtz.Fluids Barriers CNS. 2024 Sep 9;21(1):70. doi: 10.1186/s12987-024-00567-z. Fluids Barriers CNS. 2024. PMID: 39252092 Free PMC article.
Cited by
-
Intrathecal Drug Delivery: Advances and Applications in the Management of Chronic Pain Patient.Front Pain Res (Lausanne). 2022 Jun 16;3:900566. doi: 10.3389/fpain.2022.900566. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 35782225 Free PMC article. Review.
-
ABC efflux transporters at blood-central nervous system barriers and their implications for treating spinal cord disorders.Neural Regen Res. 2020 Jul;15(7):1235-1242. doi: 10.4103/1673-5374.272568. Neural Regen Res. 2020. PMID: 31960802 Free PMC article. Review.
-
Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain.J Physiol. 2018 Dec;596(23):5723-5756. doi: 10.1113/JP275376. Epub 2018 Jul 15. J Physiol. 2018. PMID: 29774535 Free PMC article. Review.
-
A journey into the brain: insight into how bacterial pathogens cross blood-brain barriers.Nat Rev Microbiol. 2017 Mar;15(3):149-159. doi: 10.1038/nrmicro.2016.178. Epub 2017 Jan 16. Nat Rev Microbiol. 2017. PMID: 28090076 Review.
-
Modeling of brain efflux: Constraints of brain surfaces.Proc Natl Acad Sci U S A. 2024 Apr 16;121(16):e2318444121. doi: 10.1073/pnas.2318444121. Epub 2024 Apr 10. Proc Natl Acad Sci U S A. 2024. PMID: 38598340 Free PMC article.
References
-
- Agrawal S., Anderson P., Durbeej M., van Rooijen N., Ivars F., Opdenakker G., et al. . (2006). Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 203, 1007–1019. 10.1084/jem.20051342 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

