A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model
- PMID: 25852489
- PMCID: PMC4365718
- DOI: 10.3389/fnana.2015.00027
A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model
Abstract
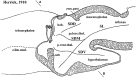
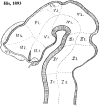
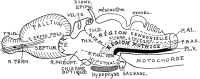
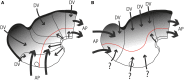
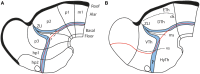
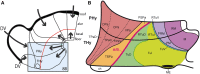
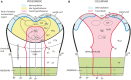
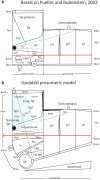
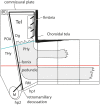
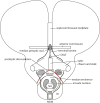
In this essay, we aim to explore in depth the new concept of the hypothalamus that was presented in the updated prosomeric model (Puelles et al., 2012b; Allen Developing Mouse Brain Atlas). Initial sections deal with the antecedents of prosomeric ideas represented by the extensive literature centered on the alternative columnar model of Herrick (1910), Kuhlenbeck (1973) and Swanson (1992, 2003); a detailed critique explores why the columnar model is not helpful in the search for causal developmental explanations. In contrast, the emerging prosomeric scenario visibly includes many possibilities to propose causal explanations of hypothalamic structure relative to both anteroposterior and dorsoventral patterning mechanisms, and insures the possibility to compare hypothalamic histogenesis with that of more caudal parts of the brain. Next the four major changes introduced in the organization of the hypothalamus on occasion of the updated model are presented, and our rationale for these changes is explored in detail. It is hoped that this example of morphological theoretical analysis may be useful for readers interested in brain models, or in understanding why models may need to change in the quest for higher consistency.
Keywords: acroterminal domain; anteroposterior pattern; dorsoventral pattern; genoarchitecture; length axis; peduncular hypothalamus; terminal hypothalamus; tracts.
Figures

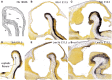
References
-
- Alvarez-Bolado G., Swanson L. W. (1996). Developmental Brain Maps: Structure of the Embryonic Rat Brain. Elsevier: Amsterdam.
-
- Altman J., Bayer S. A. (1995). Atlas of Prenatal Rat Brain Development. Boca Raton, FL: CRC Press.
LinkOut - more resources
Full Text Sources
Other Literature Sources

