IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala
- PMID: 25852553
- PMCID: PMC4365713
- DOI: 10.3389/fphar.2015.00049
IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala
Abstract
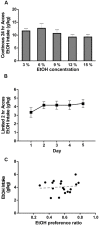
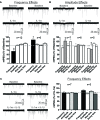
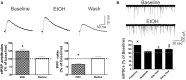
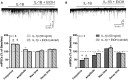
Neuroinflammation is hypothesized to enhance alcohol consumption and contribute to the development of alcoholism. GABAergic transmission in the central amygdala (CeA) plays an important role in the transition to alcohol dependence. Therefore, we studied the effects of interleukin-1β (IL-1β), a proinflammatory cytokine mediating ethanol-induced neuroinflammation, and its interaction with ethanol on CeA GABAegic transmission in B6129SF2/J mice. We also assessed ethanol intake in B6129SF2/J mice. Intake with unlimited (24 h) ethanol access was 9.2-12.7 g/kg (3-15% ethanol), while limited (2 h) access produced an intake of 4.1 ± 0.5 g/kg (15% ethanol). In our electrophysiology experiments, we found that recombinant IL-1β (50 and 100 ng/ml) significantly decreased the amplitude of evoked inhibitory postsynaptic potentials (eIPSPs), with no significant effects on paired-pulse facilitation (PPF). IL-1β (50 ng/ml) had dual effects on spontaneous miniature inhibitory postsynaptic currents (mIPSCs): increasing mIPSC frequencies in most CeA neurons, but decreasing both mIPSC frequencies and amplitudes in a few cells. The IL-1β receptor antagonist (IL-1ra; 100 ng/ml) also had dual effects on mIPSCs and prevented the actions of IL-1β on mIPSC frequencies. These results suggest that IL-1β can alter CeA GABAergic transmission at pre- and postsynaptic sites. Ethanol (44 mM) significantly increased eIPSP amplitudes, decreased PPFs, and increased mIPSC frequencies. IL-1β did not alter ethanol's enhancement of the eIPSP amplitude, but, in IL-1β-responsive neurons, the ethanol effects on mIPSC frequencies were lost. Overall, our data suggest that the IL-1 system is involved in basal GABAergic transmission and that IL-1β interacts with the ethanol-induced facilitation of CeA GABAergic transmission.
Keywords: GABAA; IL-1ra; IL-1β; IPSCs; central amygdala; cytokine; eIPSPs; interleukin.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

