Ubiquitin-fold modifier 1 acts as a positive regulator of breast cancer
- PMID: 25852645
- PMCID: PMC4367433
- DOI: 10.3389/fendo.2015.00036
Ubiquitin-fold modifier 1 acts as a positive regulator of breast cancer
Abstract
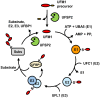
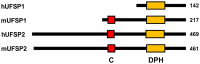
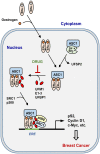
Estrogen receptor-α (ERα) is a steroid hormone-sensitive transcription factor that plays a critical role in development of breast cancer. The binding of estrogen to ERα triggers the recruitment of transcriptional co-activators as well as chromatin remodeling factors to estrogen-responsive elements (ERE) of ERα target genes. This process is tightly associated with post-translational modifications (PTMs) of ERα and its co-activators for promotion of transcriptional activation, which leads to proliferation of a large subset of breast tumor cells. These PTMs include phosphorylation, acetylation, methylation, and conjugation by ubiquitin and ubiquitin-like proteins. Ubiquitin-fold modifier 1 (UFM1), one of ubiquitin-like proteins, has recently been shown to be ligated to activating signal co-integrator 1 (ASC1), which acts as a transcriptional co-activator of nuclear receptors. Here, we discuss the mechanistic connection between ASC1 modification by UFM1 and ERα transactivation, and highlight how the interplay of these processes is involved in development of breast cancer. We also discuss potential use of UFM1-conjugating system as therapeutic targets against not only breast cancer but also other nuclear receptor-mediated cancers.
Keywords: ASC1; ERα; UFM1; breast cancer; post-translational modification.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

