Redox regulation by reversible protein S-thiolation in bacteria
- PMID: 25852656
- PMCID: PMC4360819
- DOI: 10.3389/fmicb.2015.00187
Redox regulation by reversible protein S-thiolation in bacteria
Abstract
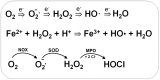
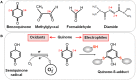
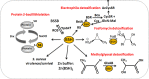
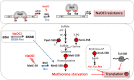
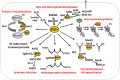
Low molecular weight (LMW) thiols function as thiol-redox buffers to maintain the reduced state of the cytoplasm. The best studied LMW thiol is the tripeptide glutathione (GSH) present in all eukaryotes and Gram-negative bacteria. Firmicutes bacteria, including Bacillus and Staphylococcus species utilize the redox buffer bacillithiol (BSH) while Actinomycetes produce the related redox buffer mycothiol (MSH). In eukaryotes, proteins are post-translationally modified to S-glutathionylated proteins under conditions of oxidative stress. S-glutathionylation has emerged as major redox-regulatory mechanism in eukaryotes and protects active site cysteine residues against overoxidation to sulfonic acids. First studies identified S-glutathionylated proteins also in Gram-negative bacteria. Advances in mass spectrometry have further facilitated the identification of protein S-bacillithiolations and S-mycothiolation as BSH- and MSH-mixed protein disulfides formed under oxidative stress in Firmicutes and Actinomycetes, respectively. In Bacillus subtilis, protein S-bacillithiolation controls the activities of the redox-sensing OhrR repressor and the methionine synthase MetE in vivo. In Corynebacterium glutamicum, protein S-mycothiolation was more widespread and affected the functions of the maltodextrin phosphorylase MalP and thiol peroxidase (Tpx). In addition, novel bacilliredoxins (Brx) and mycoredoxins (Mrx1) were shown to function similar to glutaredoxins in the reduction of BSH- and MSH-mixed protein disulfides. Here we review the current knowledge about the functions of the bacterial thiol-redox buffers glutathione, bacillithiol, and mycothiol and the role of protein S-thiolation in redox regulation and thiol protection in model and pathogenic bacteria.
Keywords: bacillithiol; glutathione; mycothiol; oxidative stress; protein S-thiolation; thiol-redox buffer.
Figures




References
-
- Ansong C., Wu S., Meng D., Liu X., Brewer H. M., Deatherage Kaiser B. L., et al. . (2013). Top-down proteomics reveals a unique protein S-thiolation switch in Salmonella Typhimurium in response to infection-like conditions. Proc. Natl. Acad. Sci. U.S.A. 110, 10153–10158. 10.1073/pnas.1221210110 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

