Intracellular oxidant activity, antioxidant enzyme defense system, and cell senescence in fibroblasts with trisomy 21
- PMID: 25852816
- PMCID: PMC4380103
- DOI: 10.1155/2015/509241
Intracellular oxidant activity, antioxidant enzyme defense system, and cell senescence in fibroblasts with trisomy 21
Abstract
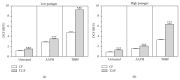
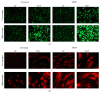
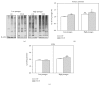
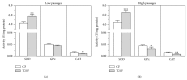
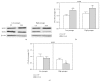
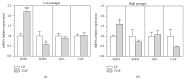
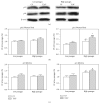
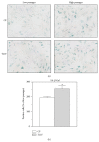
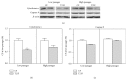
Down's syndrome (DS) is characterized by a complex phenotype associated with chronic oxidative stress and mitochondrial dysfunction. Overexpression of genes on chromosome-21 is thought to underlie the pathogenesis of the major phenotypic features of DS, such as premature aging. Using cultured fibroblasts with trisomy 21 (T21F), this study aimed to ascertain whether an imbalance exists in activities, mRNA, and protein expression of the antioxidant enzymes SOD1, SOD2, glutathione-peroxidase, and catalase during the cell replication process in vitro. T21F had high SOD1 expression and activity which led to an interenzymatic imbalance in the antioxidant defense system, accentuated with replicative senescence. Intracellular ROS production and oxidized protein levels were significantly higher in T21F compared with control cells; furthermore, a significant decline in intracellular ATP content was detected in T21F. Cell senescence was found to appear prematurely in DS cells as shown by SA-β-Gal assay and p21 assessment, though not apoptosis, as neither p53 nor the proapoptotic proteins cytochrome c and caspase 9 were altered in T21F. These novel findings would point to a deleterious role of oxidatively modified molecules in early cell senescence of T21F, thereby linking replicative and stress-induced senescence in cultured cells to premature aging in DS.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

