Fe(III) mineral reduction followed by partial dissolution and reactive oxygen species generation during 2,4,6-trinitrotoluene transformation by the aerobic yeast Yarrowia lipolytica
- PMID: 25852985
- PMCID: PMC4314830
- DOI: 10.1186/s13568-014-0094-z
Fe(III) mineral reduction followed by partial dissolution and reactive oxygen species generation during 2,4,6-trinitrotoluene transformation by the aerobic yeast Yarrowia lipolytica
Abstract
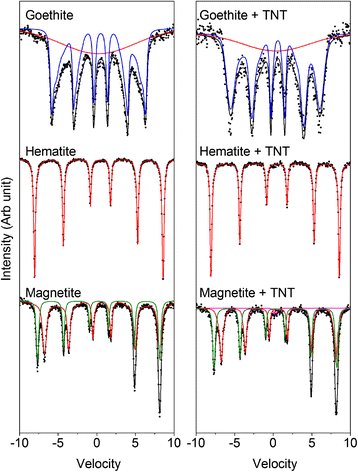
Understanding the factors that influence pollutant transformation in the presence of ferric (oxyhydr)oxides is crucial to the efficient application of different remediation strategies. In this study we determined the effect of goethite, hematite, magnetite and ferrihydrite on the transformation of 2,4,6-trinitrotoluene (TNT) by Yarrowia lipolytica AN-L15. The presence of ferric (oxyhydr)oxides led to a small decrease in the rate of TNT removal. In all cases, a significant release of NO2 (-) from TNT and further NO2 (-) oxidation to NO3 (-) was observed. A fraction of the released NO2 (-) was abiotically decomposed to NO and NO2, and then NO was likely oxidized abiotically to NO2 by O2. ESR analysis revealed the generation of superoxide in the culture medium; its further protonation at low pH resulted in the formation of hydroperoxyl radical. Presumably, a fraction of NO released during TNT degradation reacted with superoxide and formed peroxynitrite, which was further rearranged to NO3 (-) at the acidic pH values observed in this study. A transformation and reduction of ferric (oxyhydr)oxides followed by partial dissolution (in the range of 7-86% of the initial Fe(III)) were observed in the presence of cells and TNT. Mössbauer spectroscopy showed some minor changes for goethite, magnetite and ferrihydrite samples during their incubation with Y. lipolytica and TNT. This study shows that i) reactive oxygen and nitrogen species generated during TNT transformation by Y. lipolytica participate in the abiotic conversion of TNT and ii) the presence of iron(III) minerals leads to a minor decrease in TNT transformation.
Keywords: 2,4,6-Trinitrotoluene; Biodegradation; Ferric (oxyhydr)oxides; Reactive nitrogen species; Reactive oxygen species; Yarrowia lipolytica.
Figures




References
-
- Amstaetter K, Borch T, Kappler A. Influence of humic substance imposed changes of ferrihydrite aggregation on microbial Fe(III) reduction. Geochimica et Cosmochimia Acta. 2012;85:326–341. doi: 10.1016/j.gca.2012.02.003. - DOI
-
- Bielski BHJ, Cabelli DE, Arudi RL, Ross AB. Reactivity of HO2·/O2·– radicals in aqueous solution. J Phys Chem Ref Data. 1985;14:1041–1100. doi: 10.1063/1.555739. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

