[(18)F]FDG-6-P as a novel in vivo tool for imaging staphylococcal infections
- PMID: 25853019
- PMCID: PMC4385282
- DOI: 10.1186/s13550-015-0095-1
[(18)F]FDG-6-P as a novel in vivo tool for imaging staphylococcal infections
Abstract
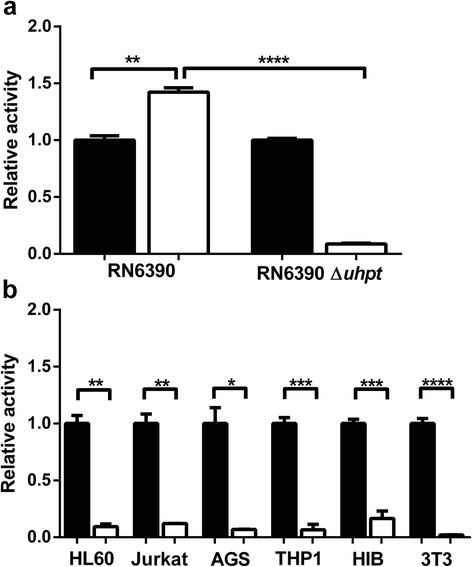
Background: Management of infection is a major clinical problem. Staphylococcus aureus is a Gram-positive bacterium which colonises approximately one third of the adult human population. Staphylococcal infections can be life-threatening and are frequently complicated by multi-antibiotic resistant strains including methicillin-resistant S. aureus (MRSA). Fluorodeoxyglucose ([(18)F]FDG) imaging has been used to identify infection sites; however, it is unable to distinguish between sterile inflammation and bacterial load. We have modified [(18)F]FDG by phosphorylation, producing [(18)F]FDG-6-P to facilitate specific uptake and accumulation by S. aureus through hexose phosphate transporters, which are not present in mammalian cell membranes. This approach leads to the specific uptake of the radiopharmaceutical into the bacteria and not the sites of sterile inflammation.
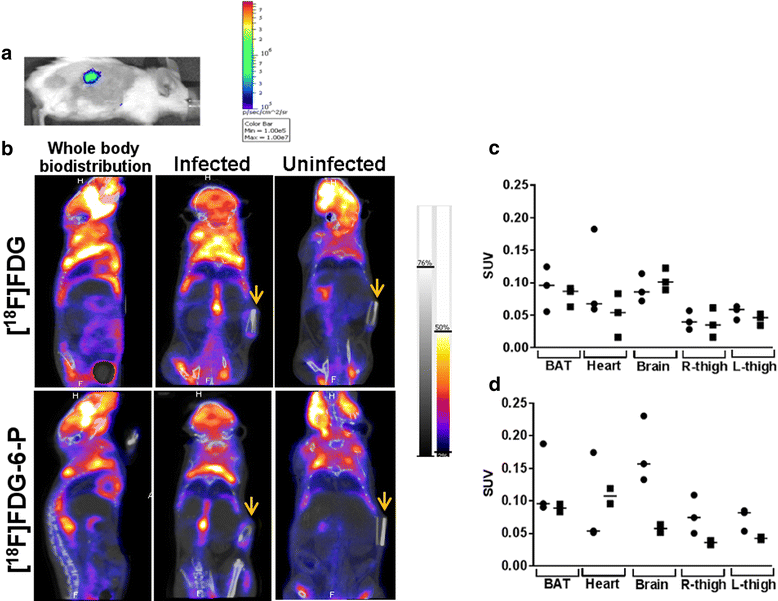
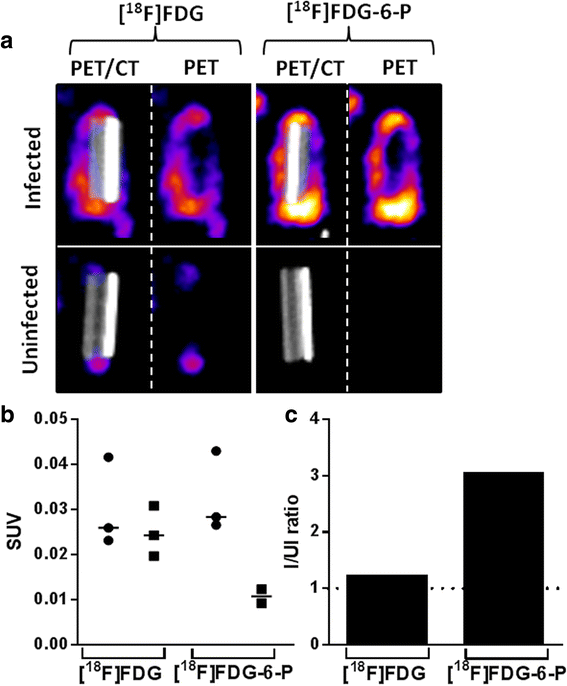
Methods: [(18)F]FDG-6-P was synthesised from [(18)F]FDG. Yield, purity and stability were confirmed by RP-HPLC and iTLC. The specificity of [(18)F]FDG-6-P for the bacterial universal hexose phosphate transporter (UHPT) was confirmed with S. aureus and mammalian cell assays in vitro. Whole body biodistribution and accumulation of [(18)F]FDG-6-P at the sites of bioluminescent staphylococcal infection were established in a murine foreign body infection model.
Results: In vitro validation assays demonstrated that [(18)F]FDG-6-P was stable and specifically transported into S. aureus but not mammalian cells. [(18)F]FDG-6-P was elevated at the sites of S. aureus infection in vivo compared to uninfected controls; however, the increase in signal was not significant and unexpectedly, the whole-body biodistribution of [(18)F]FDG-6-P was similar to that of [(18)F]FDG.
Conclusions: Despite conclusive in vitro validation, [(18)F]FDG-6-P did not behave as predicted in vivo. However at the site of known infection, [(18)F]FDG-6-P levels were elevated compared with uninfected controls, providing a higher signal-to-noise ratio. The bacterial UHPT can transport hexose phosphates other than glucose, and therefore alternative sugars may show differential biodistribution and provide a means for specific bacterial detection.
Keywords: Infection diagnosis; NanoPET-CT imaging; Pre-clinical; S. aureus.
Figures


Similar articles
-
Bacteria-targeted imaging using vancomycin-based positron emission tomography tracers can distinguish infection from sterile inflammation.Eur J Nucl Med Mol Imaging. 2025 Apr;52(5):1878-1889. doi: 10.1007/s00259-024-06997-z. Epub 2024 Nov 29. Eur J Nucl Med Mol Imaging. 2025. PMID: 39609275 Free PMC article.
-
18-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography scan for monitoring the therapeutic response in experimental Staphylococcus aureus foreign-body osteomyelitis.J Orthop Surg Res. 2015 Aug 27;10:132. doi: 10.1186/s13018-015-0274-9. J Orthop Surg Res. 2015. PMID: 26306506 Free PMC article.
-
Pilot Evaluation of S-(3-[18F]Fluoropropyl)-D-Homocysteine and O-(2-[18F]Fluoroethyl)-D-Tyrosine as Bacteria-Specific Radiotracers for PET Imaging of Infection.Mol Imaging Biol. 2024 Aug;26(4):704-713. doi: 10.1007/s11307-024-01929-7. Epub 2024 Jun 28. Mol Imaging Biol. 2024. PMID: 38942967 Free PMC article.
-
Advanced Imaging for Detection of Foci of Infection in Staphylococcus aureus Bacteremia- Can a Scan Save Lives?Semin Nucl Med. 2023 Mar;53(2):175-183. doi: 10.1053/j.semnuclmed.2023.01.002. Epub 2023 Jan 22. Semin Nucl Med. 2023. PMID: 36690574 Free PMC article. Review.
-
Pre-surgical Nasal Decolonization of Staphylococcus aureus: A Health Technology Assessment.Ont Health Technol Assess Ser. 2022 Aug 23;22(4):1-165. eCollection 2022. Ont Health Technol Assess Ser. 2022. PMID: 36160757 Free PMC article. Review.
Cited by
-
The Positron Emission Tomography Tracer 3'-Deoxy-3'-[18F]Fluorothymidine ([18F]FLT) Is Not Suitable to Detect Tissue Proliferation Induced by Systemic Yersinia enterocolitica Infection in Mice.PLoS One. 2016 Oct 4;11(10):e0164163. doi: 10.1371/journal.pone.0164163. eCollection 2016. PLoS One. 2016. PMID: 27701464 Free PMC article.
-
Microbiota in vivo imaging approaches to study host-microbe interactions in preclinical and clinical setting.Heliyon. 2022 Dec 22;8(12):e12511. doi: 10.1016/j.heliyon.2022.e12511. eCollection 2022 Dec. Heliyon. 2022. PMID: 36593827 Free PMC article. Review.
-
PET Radiopharmaceuticals for Specific Bacteria Imaging: A Systematic Review.J Clin Med. 2019 Feb 6;8(2):197. doi: 10.3390/jcm8020197. J Clin Med. 2019. PMID: 30736324 Free PMC article. Review.
-
Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade.Int J Mol Sci. 2022 Apr 30;23(9):5023. doi: 10.3390/ijms23095023. Int J Mol Sci. 2022. PMID: 35563414 Free PMC article.
-
Nuclear Imaging of Bacterial Infection: The State of the Art and Future Directions.J Nucl Med. 2020 Dec;61(12):1708-1716. doi: 10.2967/jnumed.120.244939. Epub 2020 Aug 6. J Nucl Med. 2020. PMID: 32764120 Free PMC article. Review.
References
-
- Dumarey N, Egrise D, Blocklet D, Stallenberg B, Remmelink M, del Marmol V, et al. Imaging infection with F-18-FDG-labeled leukocyte PET/CT: initial experience in 21 patients. J Nucl Med. 2006;47:625–32. - PubMed
-
- Palestro CJ, Love C, Bhargava KK. Labeled leukocyte imaging: current status and future directions. Q J Nucl Med Mol Imaging. 2009;53:105–23. - PubMed
-
- Kumar V. Radiolabeled white blood cells and direct targeting of micro-organisms for infection imaging. Q J Nucl Med Mol Imaging. 2005;49:325–38. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

