Expression and functional validation of heat-labile enterotoxin B (LTB) and cholera toxin B (CTB) subunits in transgenic rice (Oryza sativa)
- PMID: 25853032
- PMCID: PMC4380882
- DOI: 10.1186/s40064-015-0847-4
Expression and functional validation of heat-labile enterotoxin B (LTB) and cholera toxin B (CTB) subunits in transgenic rice (Oryza sativa)
Abstract
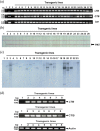
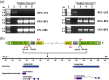
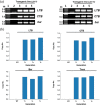
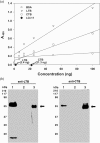
We expressed the heat-labile enterotoxin B (LTB) subunit from enterotoxigenic Escherichia coli and the cholera toxin B (CTB) subunit from Vibrio cholerae under the control of the rice (Oryza sativa) globulin (Glb) promoter. Binding of recombinant LTB and CTB proteins was confirmed based on GM1-ganglioside binding enzyme-linked immunosorbent assays (GM1-ELISA). Real-time PCR of three generations (T3, T4, and T5) in homozygous lines (LCI-11) showed single copies of LTB, CTB, bar and Tnos. LTB and CTB proteins in rice transgenic lines were detected by Western blot analysis. Immunogenicity trials of rice-derived CTB and LTB antigens were evaluated through oral and intraperitoneal administration in mice, respectively. The results revealed that LTB- and CTB-specific IgG levels were enhanced in the sera of intraperitoneally immunized mice. Similarly, the toxin-neutralizing activity of CTB and LTB in serum of orally immunized mice was associated with elevated levels of both IgG and IgA. The results of the present study suggest that the combined expression of CTB and LTB proteins can be utilized to produce vaccines against enterotoxigenic strains of Escherichia coli and Vibrio cholera, for the prevention of diarrhea.
Keywords: Enterotoxin; Immunogenicity; Oral vaccine; Oryza sativa; Transgenic plant.
Figures

References
-
- Archer DB. Enzyme production by recombinant Aspergillus. In: Murooka Y, Imanaka T, editors. Recombinant Microbes for Industrial and Agricultural Applications. New York: Marcel Dekker Inc; 1994.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

