Selective Phosphodiesterase 4B Inhibitors: A Review
- PMID: 25853062
- PMCID: PMC4318138
- DOI: 10.3797/scipharm.1404-08
Selective Phosphodiesterase 4B Inhibitors: A Review
Abstract
Phosphodiesterase 4B (PDE4B) is a member of the phosphodiesterase family of proteins that plays a critical role in regulating intracellular levels of cyclic adenosine monophosphate (cAMP) by controlling its rate of degradation. It has been demonstrated that this isoform is involved in the orchestra of events which includes inflammation, schizophrenia, cancers, chronic obstructive pulmonary disease, contractility of the myocardium, and psoriatic arthritis. Phosphodiesterase 4B has constituted an interesting target for drug development. In recent years, a number of PDE4B inhibitors have been developed for their use as therapeutic agents. In this review, an up-to-date status of the inhibitors investigated for the inhibition of PDE4B has been given so that this rich source of structural information of presently known PDE4B inhibitors could be helpful in generating a selective and potent inhibitor of PDE4B.
Keywords: Antiproliferative activity; Chronic obstructive pulmonary disease; Cyclic adenosine monophosphate; PDE4B; Phosphodiesterases (PDE) enzymes; Selective PDE inhibitors.
Figures
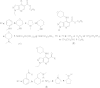
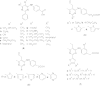
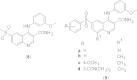
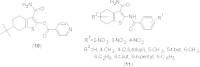
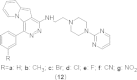
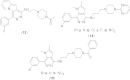

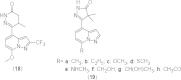
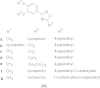
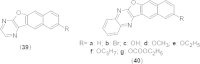
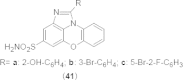
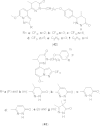
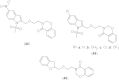
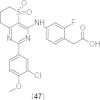
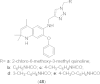
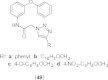
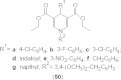
References
-
- O’Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4). Trends Pharmacol Sci. 2004;25:158–163. http://dx.doi.org/10.1016/j.tips.2004.01.003 - DOI - PubMed
-
- Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience. 2007;144:239–246. http://dx.doi.org/10.1016/j.neuroscience.2006.09.026 - DOI - PMC - PubMed
-
- Baumer W, Hoppmann J, Rundfeldt C, Kietzmann M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm Allergy Drug Targets. 2007;6:17-26. http://dx.doi.org/10.2174/187152807780077318 - DOI - PubMed
-
- Souness JE, Aldous D, Sargent C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology. 2000;47:127–162. http://dx.doi.org/10.1016/S0162-3109(00)00185-5 - DOI - PubMed
-
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. http://dx.doi.org/10.1074/jbc.R200029200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
