Neuroinflammation induced by intracerebroventricular injection of microbial neuraminidase
- PMID: 25853134
- PMCID: PMC4362343
- DOI: 10.3389/fmed.2015.00014
Neuroinflammation induced by intracerebroventricular injection of microbial neuraminidase
Abstract
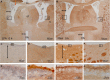
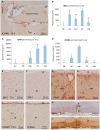
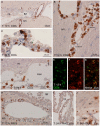
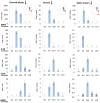
In the present paper, we describe the facts that took place in the rat brain after a single injection of the enzyme neuraminidase from Clostridium perfringens into the right lateral ventricle. After injection, it diffused through the cerebrospinal fluid of the ipsilateral ventricle and the third ventricle, and about 400 μm into the periventricular brain parenchyma. The expression of ICAM1 in the endothelial cells of the periventricular vessels, IBA1 in microglia, and GFAP in astrocytes notably increased in the regions reached by the injected neuraminidase. The subependymal microglia and the ventricular macrophages begun to express IL1β and some appeared to cross the ependymal layer. After about 4 h of the injection, leukocytes migrated from large venules of the affected choroid plexus, the meninges and the local subependyma, and infiltrated the brain. The invading cells arrived orderly: first neutrophils, then macrophage-monocytes, and last CD8α-positive T-lymphocytes and B-lymphocytes. Leukocytes in the ventricles and the perivascular zones penetrated the brain parenchyma passing through the ependyma and the glia limitans. Thus, it is likely that a great part of the damage produced by microorganism invading the brain may be due to their neuraminidase content.
Keywords: brain; ependyma; inflammation; neuraminidase; ventricle.
Figures


Similar articles
-
Neuroblast proliferation on the surface of the adult rat striatal wall after focal ependymal loss by intracerebroventricular injection of neuraminidase.J Comp Neurol. 2008 Apr 1;507(4):1571-87. doi: 10.1002/cne.21618. J Comp Neurol. 2008. PMID: 18236450
-
Ependymal denudation, aqueductal obliteration and hydrocephalus after a single injection of neuraminidase into the lateral ventricle of adult rats.J Neuropathol Exp Neurol. 1996 Sep;55(9):999-1008. doi: 10.1097/00005072-199609000-00007. J Neuropathol Exp Neurol. 1996. PMID: 8800096
-
Complement system activation contributes to the ependymal damage induced by microbial neuraminidase.J Neuroinflammation. 2016 May 21;13(1):115. doi: 10.1186/s12974-016-0576-9. J Neuroinflammation. 2016. PMID: 27209022 Free PMC article.
-
The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread.Toxicol Pathol. 2011 Jan;39(1):186-212. doi: 10.1177/0192623310394214. Epub 2010 Dec 28. Toxicol Pathol. 2011. PMID: 21189316 Review.
-
Brain iron homeostasis.Dan Med Bull. 2002 Nov;49(4):279-301. Dan Med Bull. 2002. PMID: 12553165 Review.
Cited by
-
Inducible miR-1224 silences cerebrovascular Serpine1 and restores blood flow to the stroke-affected site of the brain.Mol Ther Nucleic Acids. 2023 Jan 2;31:276-292. doi: 10.1016/j.omtn.2022.12.019. eCollection 2023 Mar 14. Mol Ther Nucleic Acids. 2023. PMID: 36726407 Free PMC article.
-
Microglial Morphometric Parameters Correlate With the Expression Level of IL-1β, and Allow Identifying Different Activated Morphotypes.Front Cell Neurosci. 2019 Oct 25;13:472. doi: 10.3389/fncel.2019.00472. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31708746 Free PMC article.
-
Microbial neuraminidase induces TLR4-dependent long-term immune priming in the brain.Front Cell Neurosci. 2022 Jul 28;16:945229. doi: 10.3389/fncel.2022.945229. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35966200 Free PMC article.
-
Altered sialidase expression in human myeloid cells undergoing apoptosis and differentiation.Sci Rep. 2022 Aug 19;12(1):14173. doi: 10.1038/s41598-022-18448-6. Sci Rep. 2022. PMID: 35986080 Free PMC article.
-
Roles of Ependymal Cells in the Physiology and Pathology of the Central Nervous System.Aging Dis. 2023 Apr 1;14(2):468-483. doi: 10.14336/AD.2022.0826-1. eCollection 2023 Apr 1. Aging Dis. 2023. PMID: 37008045 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

