The efficacy of Shugan Jianpi Zhixie therapy for diarrhea-predominant irritable bowel syndrome: a meta-analysis of randomized, double-blind, placebo-controlled trials
- PMID: 25853241
- PMCID: PMC4390216
- DOI: 10.1371/journal.pone.0122397
The efficacy of Shugan Jianpi Zhixie therapy for diarrhea-predominant irritable bowel syndrome: a meta-analysis of randomized, double-blind, placebo-controlled trials
Abstract
Background: Shugan Jianpi Zhixie therapy (SJZT) has been widely used to treat diarrhea-predominant irritable bowel syndrome (IBS-D), but the results are still controversial. A meta-analysis of randomized, double-blind, placebo-controlled trials was performed to assess the efficacy and tolerability of SJZT for IBS-D.
Methods: The MEDLINE, EMBASE, Cochrane Library, the China National Knowledge Infrastructure database, the Chinese Biomedical Literature database and the Wanfang database were searched up to June 2014 with no language restrictions. Summary estimates, including 95% confidence intervals (CI), were calculated for global symptom improvement, abdominal pain improvement, and Symptom Severity Scale (BSS) score.
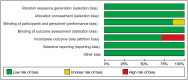
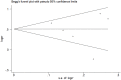
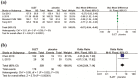
Results: Seven trials (N=954) were included. The overall risk of bias assessment was low. SJZT showed significant improvement for global symptom compared to placebo (RR 1.61; 95% CI 1.24, 2.10; P =0.0004; therapeutic gain = 33.0%; number needed to treat (NNT) = 3.0). SJZT was significantly more likely to reduce overall BSS score (SMD -0.67; 95% CI -0.94, -0.40; P < 0.00001) and improve abdominal pain (RR 4.34; 95% CI 2.64, 7.14; P < 0.00001) than placebo. The adverse events of SJZT were no different from those of placebo.
Conclusions: This meta-analysis suggests that SJZT is an effective and safe therapy option for patients with IBS-D. However, due to the high clinical heterogeneity and small sample size of the included trials, further standardized preparation, large-scale and rigorously designed trials are needed.
Conflict of interest statement
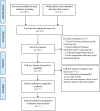
Figures

Similar articles
-
Different therapies of Chinese herbal medicine for diarrhea-predominant irritable bowel syndrome: A network meta-analysis of double-blinded, placebo-controlled trials.J Ethnopharmacol. 2023 Dec 5;317:116672. doi: 10.1016/j.jep.2023.116672. Epub 2023 Jun 14. J Ethnopharmacol. 2023. PMID: 37328079 Review.
-
Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials.Clin Ther. 2008 May;30(5):884-901. doi: 10.1016/j.clinthera.2008.05.002. Clin Ther. 2008. PMID: 18555935
-
Efficacy and safety of Modified Tongxie Yaofang in diarrhea-predominant irritable bowel syndrome management: A meta-analysis of randomized, positive medicine-controlled trials.PLoS One. 2018 Feb 6;13(2):e0192319. doi: 10.1371/journal.pone.0192319. eCollection 2018. PLoS One. 2018. PMID: 29408906 Free PMC article.
-
Therapeutic Effect of Chang'an I Recipe ( I ) on Irritable Bowel Syndrome with Diarrhea: A Multicenter Randomized Double-Blind Placebo-Controlled Clinical Trial.Chin J Integr Med. 2018 Sep;24(9):645-652. doi: 10.1007/s11655-016-2596-9. Epub 2016 Aug 3. Chin J Integr Med. 2018. PMID: 27487786 Clinical Trial.
-
Adverse events in trials of licensed drugs for irritable bowel syndrome with constipation or diarrhea: Systematic review and meta-analysis.Neurogastroenterol Motil. 2022 Jun;34(6):e14279. doi: 10.1111/nmo.14279. Epub 2021 Oct 20. Neurogastroenterol Motil. 2022. PMID: 34672052
Cited by
-
Acupuncture combined with Tongxieyaofang for diarrhea-type irritable bowel syndrome: A protocol for meta-analysis.Medicine (Baltimore). 2020 Nov 25;99(48):e23457. doi: 10.1097/MD.0000000000023457. Medicine (Baltimore). 2020. PMID: 33235133 Free PMC article.
-
An Asian perspective on irritable bowel syndrome.Intest Res. 2023 Apr;21(2):189-195. doi: 10.5217/ir.2021.00136. Epub 2022 May 31. Intest Res. 2023. PMID: 35645323 Free PMC article. Review.
-
Herbal medicines in functional dyspepsia-Untapped opportunities not without risks.Neurogastroenterol Motil. 2021 Feb;33(2):e14044. doi: 10.1111/nmo.14044. Epub 2020 Nov 30. Neurogastroenterol Motil. 2021. PMID: 33258198 Free PMC article. Review.
-
Effect of Chinese patent medicine Si-Mo-Tang oral liquid for functional dyspepsia: A systematic review and meta-analysis of randomized controlled trials.PLoS One. 2017 Feb 15;12(2):e0171878. doi: 10.1371/journal.pone.0171878. eCollection 2017. PLoS One. 2017. PMID: 28199409 Free PMC article.
-
Second Asian Consensus on Irritable Bowel Syndrome.J Neurogastroenterol Motil. 2019 Jul 1;25(3):343-362. doi: 10.5056/jnm19041. J Neurogastroenterol Motil. 2019. PMID: 31327218 Free PMC article. Review.
References
-
- Saito YA, Schoenfeld P, Locke GR 3rd (2002) The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol 97: 1910–1915. - PubMed
-
- El-Serag HB, Olden K, Bjorkman D (2002) Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther 16: 1171–1185. - PubMed
-
- Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, et al. (2005) Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care 11(Suppl 1): S17–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

