Lysosomal trafficking of TGFBIp via caveolae-mediated endocytosis
- PMID: 25853243
- PMCID: PMC4390356
- DOI: 10.1371/journal.pone.0119561
Lysosomal trafficking of TGFBIp via caveolae-mediated endocytosis
Abstract
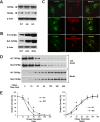
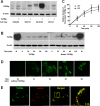
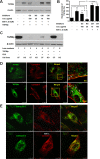
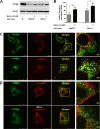
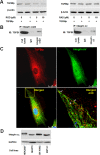
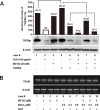
Transforming growth factor-beta-induced protein (TGFBIp) is ubiquitously expressed in the extracellular matrix (ECM) of various tissues and cell lines. Progressive accumulation of mutant TGFBIp is directly involved in the pathogenesis of TGFBI-linked corneal dystrophy. Recent studies reported that mutant TGFBIp accumulates in cells; however, the trafficking of TGFBIp is poorly understood. Therefore, we investigated TGFBIp trafficking to determine the route of its internalization and secretion and to elucidate its roles in the pathogenesis of granular corneal dystrophy type 2 (GCD2). Our data indicate that newly synthesized TGFBIp was secreted via the endoplasmic reticulum/Golgi-dependent secretory pathway, and this secretion was delayed in the corneal fibroblasts of patients with GCD2. We also found that TGFBIp was internalized by caveolae-mediated endocytosis, and the internalized TGFBIp accumulated after treatment with bafilomycin A1, an inhibitor of lysosomal degradation. In addition, the proteasome inhibitor MG132 inhibits the endocytosis of TGFBIp. Co-immunoprecipitation revealed that TGFBIp interacted with integrin αVβ3. Moreover, treatment with arginine-glycine-aspartic acid (RGD) tripeptide suppressed the internalization of TGFBIp. These insights on TGFBIp trafficking could lead to the identification of novel targets and the development of new therapies for TGFBI-linked corneal dystrophy.
Conflict of interest statement
Figures

Similar articles
-
Lysosomal dysfunction of corneal fibroblasts underlies the pathogenesis of Granular Corneal Dystrophy Type 2 and can be rescued by TFEB.J Cell Mol Med. 2020 Sep;24(18):10343-10355. doi: 10.1111/jcmm.15646. Epub 2020 Jul 15. J Cell Mol Med. 2020. PMID: 32667742 Free PMC article.
-
4-Phenylbutyric acid reduces mutant-TGFBIp levels and ER stress through activation of ERAD pathway in corneal fibroblasts of granular corneal dystrophy type 2.Biochem Biophys Res Commun. 2016 Sep 2;477(4):841-846. doi: 10.1016/j.bbrc.2016.06.146. Epub 2016 Jul 1. Biochem Biophys Res Commun. 2016. PMID: 27373828
-
Autophagy is induced by raptor degradation via the ubiquitin/proteasome system in granular corneal dystrophy type 2.Biochem Biophys Res Commun. 2014 Aug 8;450(4):1505-11. doi: 10.1016/j.bbrc.2014.07.035. Epub 2014 Jul 17. Biochem Biophys Res Commun. 2014. PMID: 25044116
-
Autophagy in granular corneal dystrophy type 2.Exp Eye Res. 2016 Mar;144:14-21. doi: 10.1016/j.exer.2015.09.008. Epub 2015 Sep 18. Exp Eye Res. 2016. PMID: 26386150 Review.
-
Pathogenesis and treatments of TGFBI corneal dystrophies.Prog Retin Eye Res. 2016 Jan;50:67-88. doi: 10.1016/j.preteyeres.2015.11.002. Epub 2015 Nov 28. Prog Retin Eye Res. 2016. PMID: 26612778 Review.
Cited by
-
Involvement of the p62/NRF2 signal transduction pathway on erythrophagocytosis.Sci Rep. 2017 Jul 19;7(1):5812. doi: 10.1038/s41598-017-05687-1. Sci Rep. 2017. PMID: 28724916 Free PMC article.
-
Lysosomal dysfunction of corneal fibroblasts underlies the pathogenesis of Granular Corneal Dystrophy Type 2 and can be rescued by TFEB.J Cell Mol Med. 2020 Sep;24(18):10343-10355. doi: 10.1111/jcmm.15646. Epub 2020 Jul 15. J Cell Mol Med. 2020. PMID: 32667742 Free PMC article.
-
Endoplasmic reticulum stress: molecular mechanism and therapeutic targets.Signal Transduct Target Ther. 2023 Sep 15;8(1):352. doi: 10.1038/s41392-023-01570-w. Signal Transduct Target Ther. 2023. PMID: 37709773 Free PMC article. Review.
-
Actinobacillus pleuropneumoniae Interaction With Swine Endothelial Cells.Front Vet Sci. 2020 Oct 29;7:569370. doi: 10.3389/fvets.2020.569370. eCollection 2020. Front Vet Sci. 2020. PMID: 33195549 Free PMC article.
-
Molecular genetic analysis of R124H TGFBIp in one family Avellino corneal dystrophy.Mol Vis. 2024 Sep 30;30:290-297. eCollection 2024. Mol Vis. 2024. PMID: 39959167 Free PMC article.
References
-
- Munier FL, Korvatska E, Djemai A, Le Paslier D, Zografos L, Pescia G, et al. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet. 1997;15: 247–251. - PubMed
-
- Klintworth GK. Advances in the molecular genetics of corneal dystrophies. Am J Ophthalmol. 1999;128: 747–754. - PubMed
-
- Munier FL, Frueh BE, Othenin-Girard P, Uffer S, Cousin P, Wang MX, et al. BIGH3 mutation spectrum in corneal dystrophies. Invest Ophthalmol Vis Sci. 2002;43: 949–954. - PubMed
-
- Yuan C, Yang MC, Zins EJ, Boehlke CS, Huang AJ. Identification of the promoter region of the human betaIGH3 gene. Mol Vis. 2004;10: 351–360. - PubMed
-
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99: 81–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

