PGC-1α activity in nigral dopamine neurons determines vulnerability to α-synuclein
- PMID: 25853296
- PMCID: PMC4379693
- DOI: 10.1186/s40478-015-0200-8
PGC-1α activity in nigral dopamine neurons determines vulnerability to α-synuclein
Abstract
Introduction: Mitochondrial dysfunction and oxidative stress are critical factors in the pathogenesis of age-dependent neurodegenerative diseases. PGC-1α, a master regulator of mitochondrial biogenesis and cellular antioxidant defense, has emerged as a possible therapeutic target for Parkinson's disease, with important roles in the function and survival of dopaminergic neurons in the substantia nigra. The objective of this study is to determine if the loss of PGC-1α activity contributes to α-synuclein-induced degeneration.
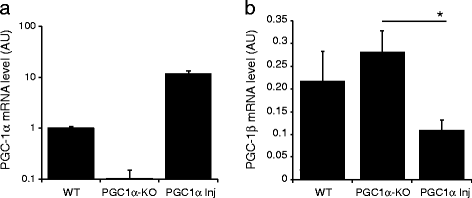
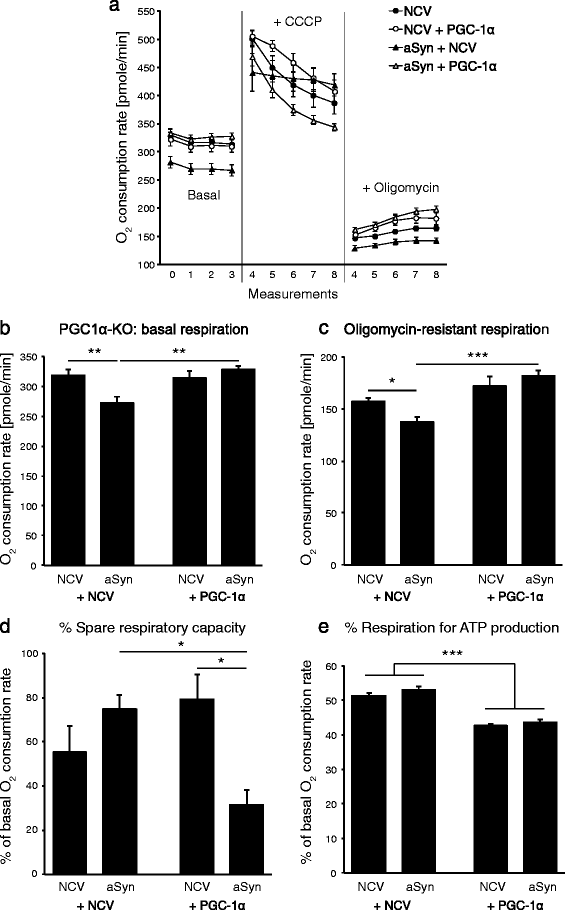
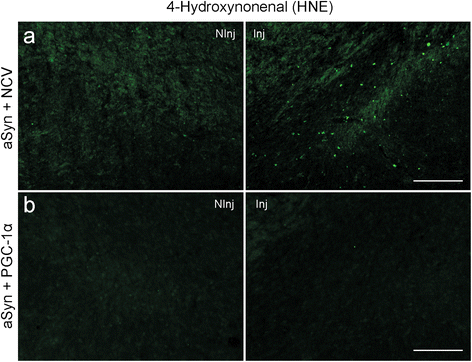
Results: We explore the vulnerability of PGC-1α null mice to the accumulation of human α-synuclein in nigral neurons, and assess the neuroprotective effect of AAV-mediated PGC-1α expression in this experimental model. Using neuronal cultures derived from these mice, mitochondrial respiration and production of reactive oxygen species are assessed in conditions of human α-synuclein overexpression. We find ultrastructural evidence for abnormal mitochondria and fragmented endoplasmic reticulum in the nigral dopaminergic neurons of PGC-1α null mice. Furthermore, PGC-1α null nigral neurons are more prone to degenerate following overexpression of human α-synuclein, an effect more apparent in male mice. PGC-1α overexpression restores mitochondrial morphology, oxidative stress detoxification and basal respiration, which is consistent with the observed neuroprotection against α-synuclein toxicity in male PGC-1α null mice.
Conclusions: Altogether, our results highlight an important role for PGC-1α in controlling the mitochondrial function of nigral neurons accumulating α-synuclein, which may be critical for gender-dependent vulnerability to Parkinson's disease.
Figures






Similar articles
-
Sustained expression of PGC-1α in the rat nigrostriatal system selectively impairs dopaminergic function.Hum Mol Genet. 2012 Apr 15;21(8):1861-76. doi: 10.1093/hmg/ddr618. Epub 2012 Jan 12. Hum Mol Genet. 2012. PMID: 22246294 Free PMC article.
-
Promotion of mitochondrial biogenesis by necdin protects neurons against mitochondrial insults.Nat Commun. 2016 Mar 14;7:10943. doi: 10.1038/ncomms10943. Nat Commun. 2016. PMID: 26971449 Free PMC article.
-
REST Protects Dopaminergic Neurons from Mitochondrial and α-Synuclein Oligomer Pathology in an Alpha Synuclein Overexpressing BAC-Transgenic Mouse Model.J Neurosci. 2021 Apr 21;41(16):3731-3746. doi: 10.1523/JNEUROSCI.1478-20.2021. Epub 2021 Feb 9. J Neurosci. 2021. PMID: 33563726 Free PMC article.
-
PGC-1s in the Spotlight with Parkinson's Disease.Int J Mol Sci. 2021 Mar 28;22(7):3487. doi: 10.3390/ijms22073487. Int J Mol Sci. 2021. PMID: 33800548 Free PMC article. Review.
-
PGC-1α: a master gene that is hard to master.Cell Mol Life Sci. 2012 Aug;69(15):2465-8. doi: 10.1007/s00018-012-1043-0. Epub 2012 Jun 9. Cell Mol Life Sci. 2012. PMID: 22678664 Free PMC article. Review.
Cited by
-
Neuromedin S Regulates Steroidogenesis through Maintaining Mitochondrial Morphology and Function via NMUR2 in Goat Ovarian Granulosa Cells.Int J Mol Sci. 2022 Nov 2;23(21):13402. doi: 10.3390/ijms232113402. Int J Mol Sci. 2022. PMID: 36362185 Free PMC article.
-
dVGLUT Is a Mediator of Sex Differences in Dopamine Neuron Mitochondrial Function Across Aging and in a Parkinson's Disease Model.Aging Cell. 2025 Aug;24(8):e70096. doi: 10.1111/acel.70096. Epub 2025 May 12. Aging Cell. 2025. PMID: 40354193 Free PMC article.
-
Mechanisms of mitochondrial respiratory adaptation.Nat Rev Mol Cell Biol. 2022 Dec;23(12):817-835. doi: 10.1038/s41580-022-00506-6. Epub 2022 Jul 8. Nat Rev Mol Cell Biol. 2022. PMID: 35804199 Free PMC article. Review.
-
Adult Conditional Knockout of PGC-1α Leads to Loss of Dopamine Neurons.eNeuro. 2016 Sep 2;3(4):ENEURO.0183-16.2016. doi: 10.1523/ENEURO.0183-16.2016. eCollection 2016 Jul-Aug. eNeuro. 2016. PMID: 27622213 Free PMC article.
-
Mitochondrial Dysfunction in Parkinson's Disease: New Mechanistic Insights and Therapeutic Perspectives.Curr Neurol Neurosci Rep. 2018 Apr 3;18(5):21. doi: 10.1007/s11910-018-0829-3. Curr Neurol Neurosci Rep. 2018. PMID: 29616350 Free PMC article. Review.
References
-
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38(5):515–517. doi: 10.1038/ng1769. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

